Abstract
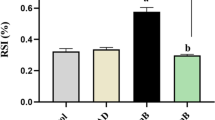
Lipopolysaccharide (LPS) from bacteria can accelerate and exacerbate lupus nephritis (LN) and induce infiltrating inflammatory cells in kidney in animal models. Pyrrolidine dithiocarbamate (PDTC) is known to exert anti-inflammatory effects. Monocyte chemoattractant protein-1(MCP-1) is upregulated by various stimuli, including LPS, high glucose, and hyperosmolality. However, the molecular mechanisms of transcriptional regulation of the MCP-1 protein expression with LPS are poorly understood. Expression of MCP-1 was examined by western blot and enzyme-linked immunosorbent assay, respectively. The activity of nuclear factor (NF)-kappaB was measured by western blot. These mice have uncontrolled proliferation of T cells, an impaired response to T cell mitogen and produce autoantibodies against nuclear antigens, including DNA. We found that after LPS treatment for 14 weeks, LPS increased MCP-1 protein expression in kidney, which was significantly suppressed by antioxidant PDTC. The expression of NF-κB, pERK, pJNK and MCP-1 were increased, pp38 expression was decreased significantly, concomitantly with sera anti-dsDNA, MCP-1 and the acceleration of severity of autoimmune kidney injury. LPS induce markedly neutrophil infiltration in the glomerulus, especially around the mesangial region. PDTC reduced the number of infiltrating inflammatory cells and severity of kidney injury via inhibiting NF-κB and p38 MAPK activity. They also markedly prevented LPS-induced pJNK and MCP-1. Therefore, MCP-1 may be responsible for the recruitment and activation of leukocytes in diseased kidneys in female MRL/lpr mice. In this study, the long-term administration of PDTC had impacts on the prevention of end-stage organ damage induced by LPS treated. We demonstrated that PDTC inhibited LPS-induced monocyte migration and attenuated LPS-induced p38 MAPK activation. Based on these data we infer that PDTC inhibits LPS-induced MCP-1 expression, secretion and function through inhibition of NF-κB and p38 MAPK activity. Our study suggests that MAPK is an important therapeutic target of monocyte recruitment and accumulation within the glomerulus in inflammatory renal disease. These results suggest that PDTC protects against kidney inflammation of SLE at least in part via NF-κB and MAPK signaling pathways induction, and that inhibitory action on anti-dsDNA may be associated with the protective mechanism of PDTC. In summary, PDTC pretreatment attenuates LPS-induced kidney injury in female MRL/lpr mice through regulating NF-κB and MAPK signaling pathways. Our results indicate that LPS induces MCP-1 mainly through activating NF-κB and its downstream MAPK, and that such effect was inhibited by PDTC, suggesting the efficacy of PDTC in preventing kidney fibrosis in lupus-prone mice. Therefore, appropriate inhibition of NF-κB activation may attenuate the kidney injury in lupus-prone mice.




Similar content being viewed by others
References
Wakeland EK, Liu K, Graham RR, Behrens TW (2001) Delineating the genetic basis of systemic lupus erythematosus. Immunity 15:397–408
Poole BD, Scofield RH, Harley JB, James JA (2006) Epstein-Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity 39:63–70
Zandman-Goddard G, Shoenfeld Y (2005) Infections and SLE. Autoimmunity 38:473–485
Baechlerl EC, Gregersen PK, Behrens TW (2004) The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol 16:801–807
Tveita Anders, Rekvig PO, Zykova EN (2008) Glomerular matrix metalloproteinases and their regulators in the pathogenesis of lupus nephritis. Arthritis Res Ther 10:229
Kalergis AM, Iruretagoyena MI, Barrientos MJ, González PA, Herrada AA, Leiva ED, Gutiérrez MA, Riedel CA, Bueno SM, Jacobelli SH (2009) Modulation of nuclear factor-kappaB activity can influence the susceptibility to systemic lupus erythematosus. Immunology 128(1 Suppl):e306–e314
Ho SY, Wu WJ, Chiu HW, Chen YA, Ho YS, Guo HR, Wang YJ (2011) Arsenic trioxide and radiation enhance apoptotic effects in HL-60 cells through increased ROS generation and regulation of JNK and p38 MAPK signaling pathways. Chem Biol Interact 193(2):162–171
Lee ER, Kim JH, Choi HY, Jeon K, Cho SG (2011) Cytoprotective effect of eriodictyol in UV-irradiated keratinocytes via phosphatase-dependent modulation of both the p38 MAPK and Akt signaling pathways. Cell Physiol Biochem 27(5):513–524
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11:183–190
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M (2005) IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198
Viedt C, Dechend R, Fei J, Hansch GM, Kreuzer J, Orth SR (2002) MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol 13:1534–1547
Massague J, Blain SW, Lo RS (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103:295–309
Manson JJ, Ma A, Rogers P, Mason LJ, Berden JH, van der Vlag J, D’Cruz DP, Isenberg DA, Rahman A (2009) Relationship between anti-dsDNA, anti-nucleosome and anti-alpha-actinin antibodies and markers of renal disease in patients with lupus nephritis: a prospective longitudinal study. Arthritis Res Ther 11(5):R154
Hahn BH (1998) Antibodies to DNA. N Engl J Med 338:1359–1368
Skaggs BJ, Lourenço EV, Hahn BH (2011) Oral administration of different forms of a tolerogenic peptide to define the preparations and doses that delay anti-DNA antibody production and nephritis and prolong survival in SLE-prone mice. Lupus 20(9):912–920
Mostoslavsky G, Fischel R, Yachimovich N, Yarkoni Y, Rosenmann E, Monestier M, Baniyash M, Eilat D (2001) Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur J Immunol 31:1221–1227
Katz JB, Limpanasithikul W, Diamond B (1994) Mutational analysis of an autoantibody: differential binding and pathogenicity. J Exp Med 180:925–932
Keiichiro Matoba, Daiji Kawanami, Ishizawa Sho, Kanazawa Yasushi, Yokota Tamotsu, Utsunomiya Kazunori (2010) Rho-kinase mediates TNF-a-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun 402:725–730
Sime PJ, O’Reilly KM (2001) Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol 3:308–319
Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J (2002) MAPKK-independent activation of p38alpha mediated by TAB 1-dependent autophosphorylation of p38alpha. Science 295:1291–1294
Berden JH (1997) Lupus nephritis. Kidney Int 52:538–558
Alexander JJ, Zwingmann C, Alexander C, Quigg R (2007) Alteration in kidney glucose and amino acids are implicated in renal pathology in MRL/lpr mice. Biochim Biophys Acta 1772:1143–1149
Kiani AN, Johnson K, Chen C, Diehl E, Hu H, Vasudevan G, Singh S, Magder LS, Knechtle SJ, Petri M (2009) Urine osteoprotegerin and monocyte chemoattractant protein-1 in lupus nephritis. J Rheumatol 36(10):2224–2230
Shimizu S, Nakashima H, Masutani K, Inoue Y, Miyake K, Akahoshi M, Tanaka Y, Egashira K, Hirakata H, Otsukal T, Harada M (2004) Anti-monocyte chemoattractant protein-1 gene therapy attenuates nephritis in MRL/lpr mice. Rheumatology 43:1121–1128
Odobasic D, Kitching AR, Semple TJ, Timoshanko JR, Tipping PG, Holdsworth SR (2005) Glomerular expression of CD80 and CD86 is required for leukocyte accumulation and injury in crescentic glomerulonephritis. J Am Soc Nephrol 16(7):2012–2022
Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH (2004) Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int 65:116–128
Galkina E, Ley K (2006) Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 17:368–377
Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR (2009) Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J Am Soc Nephrol 20(12):2581–2592
Docherty NG, O’Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW (2006) Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290:F4–F13
Liu Y (2006) Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69:213–217
Zhi-Chun L, Qiao-Ling Z, Zhi-Qin L, Xiao-Zhao L, Xiao-Xia Z, Rong T (2011) Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) mediates p38 mitogen-activated protein kinase activation and signal transduction in peripheral blood mononuclear cells from patients with lupus nephritis. Inflammation. doi:10.1007/s10753-011-9396-3
Marks SD, Shah V, Pilkington C, Tullus K (2010) Urinary monocyte chemoattractant protein-1 correlates with disease activity in lupus nephritis. Pediatr Nephrol 25(11):2283–2288
Acknowledgments
We thank Prof. Yuan Wang for his reliable assistance in performing western blots. This work was partly supported by Grants from the key program of the National Natural Science Foundation of China (30830089).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhai, JX., Zhang, ZX., Feng, YJ. et al. PDTC attenuate LPS-induced kidney injury in systemic lupus erythematosus-prone MRL/lpr Mice. Mol Biol Rep 39, 6763–6771 (2012). https://doi.org/10.1007/s11033-012-1501-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1501-7