Abstract
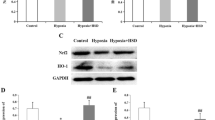
The aim of this study was to investigate the changes of SDF-1α and ILK expression in mouse retinal pigment epithelium (RPE) cells in response to hypoxia, and the effect of 17-Allylamino-17-demethoxygeldanamycin (17-AAG), a heat shock protein 90 (Hsp90) inhibitor, on the hypoxia-induced expression of SDF-1α and ILK. RPE cells were cultured with 200 μmol/L cobalt chloride (CoCl2) for different times (1, 3, 6, 12, 24, 72 h) to imitate chemical hypoxia. Pretreatment of 17-AAG was 1 h prior to hypoxic insult. Cellular viability after 17-AAG treatment was assessed by MTT assay, and the changes of SDF-1α and ILK expression were examined by RT-PCR and Western blot. Up-regulation of SDF-1α and ILK expression in response to hypoxia was observed. One hour pretreatment of 17-AAG could remarkably decreased the hypoxia-induced SDF-1α and ILK expression in vitro. Our results indicated that SDF-1α and ILK involved in the hypoxic response of RPE cells, and 1 h pretreatment of 17-AAG had an inhibitive effect on the hypoxia-induced SDF-1α and ILK expression.



Similar content being viewed by others
Abbreviations
- Hsp90:
-
Heat shock protein 90
- RPE:
-
Retinal pigment epithelium
- EPCs:
-
Endothelium progenitor cells
References
Csaky KG, Baffi JZ, Byrnes GA et al (2004) Recruitment of marrow-derived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exp Eye Res 78:1107–1116. doi:10.1016/j.exer.2004.01.010
Shao H, Tan Y, Eton D et al (2008) Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells 26:1376–1384. doi:10.1634/stemcells.2007-0785
Butler JM, Guthrie SM, Koc M et al (2005) SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 115:86–93
Sengupta N, Caballero S, Mames RN et al (2005) Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci 46:343–348. doi:10.1167/iovs.04-0153
McDonald PC, Oloumi A, Mills J et al (2008) Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res 68:1618–1624. doi:10.1158/0008-5472.CAN-07-5869
Cho HJ, Youn SW, Cheon SI et al (2005) Regulation of endothelial cell and endothelial progenitor cell survival and vasculogenesis by integrin-linked kinase. Arterioscler Thromb Vasc Biol 25:1154–1160. doi:10.1161/01.ATV.0000164312.20008.93
Hannigan G, Troussard AA, Dedhar S (2005) Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer 5:51–63. doi:10.1038/nrc1524
Friedrich EB, Liu E, Sinha S et al (2004) Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol 24:8134–8144. doi:10.1128/MCB.24.18.8134-8144.2004
Lee SP, Youn SW, Cho HJ et al (2006) Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation 114:150–159. doi:10.1161/CIRCULATIONAHA.105.595918
Li YJ, Hui YN, Yan F et al (2007) Up-regulation of integrin-linked kinase in the streptozotocin-induced diabetic rat retina. Graefes Arch Clin Exp Ophthalmol 245:1523–1532. doi:10.1007/s00417-007-0616-3
Furth AF, Mandrekar SJ, Tan AD et al (2008) A limited sample model to predict area under the drug concentration curve for 17-(allylamino)-17-demethoxygeldanamycin and its active metabolite 17-(amino)-17-demethoxygeldanomycin. Cancer Chemother Pharmacol 61:39–45. doi:10.1007/s00280-007-0443-6
Kociok N, Krohne TU, Poulaki V et al (2007) Geldanamycin treatment reduces neovascularization in a mouse model of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 245:258–266. doi:10.1007/s00417-006-0355-x
Zamiri P, Masli S, Kitaichi N et al (2005) Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci 46:908–919. doi:10.1167/iovs.04-0362
Fine SL (2005) Age-related macular degeneration 1969–2004: a 35-year personal perspective. Am J Ophthalmol 139:405–420. doi:10.1016/j.ajo.2004.11.050
Maberley DA, Hollands H, Chuo J et al (2006) The prevalence of low vision and blindness in Canada. Eye 20:341–346. doi:10.1038/sj.eye.6701879
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881. doi:10.1152/physrev.00021.2004
Crane IJ, Wallace CA, McKillop-Smith S et al (2000) CXCR4 receptor expression on human retinal pigment epithelial cells from the blood-retina barrier leads to chemokine secretion and migration in response to stromal cell-derived factor 1 alpha. J Immunol 165:4372–4378
Eichler W, Reiche A, Yafai Y et al (2008) Growth-related effects of oxidant-induced stress on cultured RPE and choroidal endothelial cells. Exp Eye Res 87:342–348. doi:10.1016/j.exer.2008.06.017
Yu L, Cecil J, Peng SB et al (2006) Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene 374:174–179. doi:10.1016/j.gene.2006.02.001
Li Y, Reca RG, Atmaca-Sonmez P et al (2006) Retinal pigment epithelium damage enhances expression of chemoattractants and migration of bone marrow-derived stem cells. Invest Ophthalmol Vis Sci 47:1646–1652. doi:10.1167/iovs.05-1092
Scandurro AB, Weldon CW, Figueroa YG et al (2001) Gene microarray analysis reveals a novel hypoxia signal transduction pathway in human hepatocellular carcinoma cells. Int J Oncol 19:129–135
Edwards LA, Woo J, Huxham LA et al (2008) Suppression of VEGF secretion and changes in glioblastoma multiforme microenvironment by inhibition of integrin-linked kinase (ILK). Mol Cancer Ther 7:59–70. doi:10.1158/1535-7163.MCT-07-0329
Tan C, Cruet-Hennequart S, Troussard A et al (2004) Regulation of tumor angiogenesis by integrin-linked kinase (ILK). Cancer Cell 5:79–90. doi:10.1016/S1535-6108(03)00281-2
Egorin MJ, Zuhowski EG, Rosen DM et al (2001) Plasma pharmacokinetics and tissue distribution of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) in CD2F1 mice1. Cancer Chemother Pharmacol 47:291–302. doi:10.1007/s002800000242
Wu WC, Kao YH, Hu PS et al (2007) Geldanamycin, a HSP90 inhibitor, attenuates the hypoxia-induced vascular endothelial growth factor expression in retinal pigment epithelium cells in vitro. Exp Eye Res 85:721–731. doi:10.1016/j.exer.2007.08.005
Kryczek I, Lange A, Mottram P et al (2005) CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res 65:465–472
Acknowledgments
The authors wish to thank all the participants and researchers who took participation in the study. This work was supported by department of Biopharmaceutical Sciences, College of Pharmacy, Harbin Medical University and Harbin Engineering University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y.Q., Zhang, X.M., Wang, X.D. et al. 17-AAG, a Hsp90 inhibitor, attenuates the hypoxia-induced expression of SDF-1α and ILK in mouse RPE cells. Mol Biol Rep 37, 1203–1209 (2010). https://doi.org/10.1007/s11033-009-9490-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9490-x