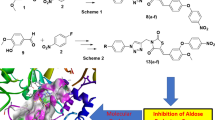
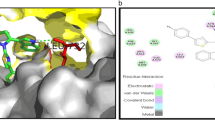
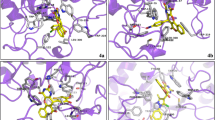
Abstract
Thiazolidinedione has been used successfully by medicinal chemists all over the world in the development of potent antidiabetic derivatives. The few compounds with excellent antidiabetic potency that we have identified in this review could be used as a lead for further research into additional antidiabetic mechanisms. The information provided in this review regarding the design, biological activity, structure–activity relationships, and docking studies may be useful for scientists who wish to further explore this scaffold in order to fully utilize its biological potential and develop antidiabetic agents that would overcome the limitations of currently available medications for the treatment of diabetes. This review outlines the antidiabetic potential of Thiazolidinedione-based derivatives that have been published in the year 2021- till date.
Graphical Abstract
























Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine Transaminase
- AST:
-
Aspartate Transaminase
- ADMET:
-
Absorption, Distribution, Metabolism, Excretion and Toxicity
- ALR:
-
Aldose reductase
- DPP-4:
-
Dipeptidyl peptidase-4
- D.B:
-
Direct Bilirubin
- DEN:
-
Diethylnitrosamine
- ELISA:
-
Enzyme-linked immunosorbent assay
- GSH:
-
Glutathione
- GA-MLR:
-
Genetic Algorithm Multiple Linear Regression
- HSA:
-
Human Serum albumin
- IC50:
-
Half-maximal inhibitory concentration
- IR:
-
Infrared Spectroscopy
- I.D.B:
-
Indirect Bilirubin
- LDL:
-
Low density lipoproteins
- LFT:
-
Liver Function test
- LDH:
-
Lactate Dehydrogenase
- MM-GBSA:
-
Molecular Mechanics-Generalized Born Surface area
- MDA:
-
Malondialdehyde
- NMR:
-
Nuclear Magnetic Resonance Spectroscopy
- OGTT:
-
Oral Glucose tolerance test
- PPAR-γ :
-
Peroxisome proliferator Activated receptors
- PTP-1B:
-
Protein tyrosine phosphatase 1B
- PDB:
-
Protein Database
- QSAR:
-
Quantitative Structure Activity Relationship
- RSG:
-
Rosiglitazone
- SPR:
-
Surface plasmon resonance
- STZ:
-
Streptozocin
- STZ-NA:
-
Streptozotocin-nicotinamide
- SOD:
-
Superoxide dismutase
- TZD:
-
Thiazolidinedione
- TG:
-
Triglycerides
- TR-FRET:
-
Time-resolved fluorescence resonance energy transfer
- T.B.:
-
Total Bilirubin
- Z5-2 T:
-
(Z)-5(pyridine-2-ylmethylene)-2-thioxothiazolidin-4-one
References
Fal M, El-Fal M, Sayah K et al (2017) Synthesis, and evaluation of α-amylase and α-glucosidase inhibitory potential of new pyrazolo [3,4-d]pyrimidine derivatives. Eur J Chem 8:105–108. https://doi.org/10.5155/eurjchem.8.2.105-108.1541
American Diabetes Association (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes care. https://doi.org/10.2337/dc20-S002
IDF Diabetes Atlas International Diabetes Federation (2023) Google Scholar. https://scholar.google.com/scholar?q=IDF+Diabetes+Atlas+International+Diabetes+Federation+Brussels,+Belgium+International+Diabetes+Federation+2021+https://idf.org/e-library/epidemiology-research/diabetes-atlas.html+. Accessed 20 Oct 2023
Hegazi R, El-Gamal M, Abdel-Hady N, Hamdy O (2015) Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann Glob Health 81:814–820. https://doi.org/10.1016/j.aogh.2015.12.011
Hajji S, Aljenaee K, Garrahy A, Byrne M (2021) Successful transition from insulin to sulfonylurea, on second attempt, in a 24-year-old female with neonatal diabetes secondary to KCNJ11 gene mutation. BMJ Case Rep 14:e239973
Mifsud S, Schembri EL, Fava S (2019) A case of severe relapsing sulphonylurea-induced hypoglycaemia. BMJ Case Rep 12:e231368
Sartore G, Ragazzi E, Caprino R, Lapolla A (2023) Long-term HbA1c variability and macro-/micro-vascular complications in type 2 diabetes mellitus: a meta-analysis update. Acta Diabetol. https://doi.org/10.1007/s00592-023-02037-8
Doyle-Delgado K, Chamberlain JJ, Shubrook JH, Skolnik N, Trujillo J (2020) Pharmacologic approaches to glycemic treatment of type 2 diabetes: synopsis of the 2020 American diabetes association’s standards of medical care in diabetes clinical guideline. Ann Intern Med 173:813–821
Rakhis Sr SAB, AlDuwayhis NM, Aleid N et al (2022) Glycemic control for type 2 diabetes mellitus patients: a systematic review. Cureus. https://doi.org/10.7759/CUREUS.26180
Lebovitz HE (2019) Thiazolidinediones: the forgotten diabetes medications. Curr Diab Rep. https://doi.org/10.1007/S11892-019-1270-Y
Long N, Le Gresley A, Wren SP (2021) Thiazolidinediones: an in–depth study of their synthesis and application to medicinal chemistry in the treatment of diabetes mellitus. ChemMedChem 16:1717. https://doi.org/10.1002/CMDC.202100177
Ahn S, Lee M, An S et al (2018) 2-Formyl-komarovicine promotes adiponectin production in human mesenchymal stem cells through PPARγ partial agonism. Bioorg Med Chem 26:1069–1075. https://doi.org/10.1016/J.BMC.2018.01.019
Lehmann JM, Moore LB, Smith-Oliver TA et al (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ). J Biol Chem 270:12953–12956. https://doi.org/10.1074/jbc.270.22.12953
Dirven H, Vist GE, Bandhakavi S, Mehta J, Fitch SE, Pound P, Ram R, Kincaid B, Leenaars CHC, Chen M, Wright RA, Tsaioun K (2021) Performance of preclinical models in predicting drug-induced liver injury in humans: a systematic review. Sci Rep 11:6403. https://doi.org/10.1038/s41598-021-85708-2
Babai S, Auclert L, Le-Louet H (2021) Safety data and withdrawal of hepatotoxic drugs. Therapie 76:715–723. https://doi.org/10.1016/j.therap.2018.02.004
Mannucci E, Dicembrini I (2015) Drugs for type 2 diabetes: role in the regulation of bone metabolism. Clin Cases Miner Bone Metab. https://doi.org/10.11138/CCMBM/2015.12.2.130
Good AC, Liu J, Hirth B, Asmussen G, Xiang Y, Biemann HP, Bishop KA, Fremgen T, Fitzgerald M, Gladysheva T, Jain A, Jancsics K, Metz M, Papoulis A, Skerlj R, Stepp JD, Wei RR (2012) J Med Chem 55:2641–2648
Mueller SL, Chrysanthopoulos PK, Halili MA et al (2021) The glitazone class of drugs as carbonic anhydrase inhibitors—a spin-off discovery from fragment screening. Molecules. https://doi.org/10.3390/MOLECULES26103010
Shang J, Brust R, Griffin PR, Kamenecka TM, Kojetin DJ (2019) Quantitative structural assessment of graded receptor agonism. Proc Natl Acad Sci 116(44):22179–22188
Chrysanthopoulos PK, Mujumdar P, Woods LA, Dolezal O, Ren B, Peat TS, Poulsen SA (2017) Identification of a new zinc binding chemotype by fragment screening. J Med Chem 60(17):7333–7349
Kumar H, Aggarwal N, Marwaha MG, Deep A, Chopra H, Matin MM, Al-Harrasi A (2022) Thiazolidin-2, 4-dione scaffold: an insight into recent advances as antimicrobial, antioxidant, and hypoglycemic agents. Molecules 27(19):6763
Soni HI, Patel NB, Parmar RB, Chan-Bacab MJ, Rivera G (2022) Microwave irradiated synthesis of pyrimidine containing, thiazolidin-4-ones: antimicrobial, anti-tuberculosis, antimalarial and anti-protozoa evaluation. Lett Org Chem 19(9):731–738
Dreyer C, Krey G, Keller H et al (1992) Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68:879–887. https://doi.org/10.1016/0092-8674(92)90031-7
Schouten H, Klitgord KD, Whitehead JA, Macdonald KC (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347(6294):645–650
Meirhaeghe A, Amouyel P (2004) Impact of genetic variation of PPAR in humans. Mol Genet Metab 83:93–102. https://doi.org/10.1016/j.ymgme.2004.08.014
Zoete V, Grosdidier A, Michielin O (2007) Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim et Biophys Acta (BBA)—Mol Cell Biol Lipids. https://doi.org/10.1016/j.bbalip.2007.01.007
Chittiboyina AG, Venkatraman MS, Mizuno CS et al (2006) Design and synthesis of the first generation of dithiolane thiazolidinedione-and phenylacetic acid-based PPARγ agonists. J Med Chem. https://doi.org/10.1021/jm0510880
Madhavan GR, Chakrabarti R, Vikramadithyan RK et al (2002) Synthesis and biological activity of novel pyrimidinone containing thiazolidinedione derivatives. Bioorg Med Chem 10(8):2671–2680
Naim MJ, Alam O, Alam MJ et al (2018) Synthesis, docking, in vitro and in vivo antidiabetic activity of pyrazole-based 2,4-thiazolidinedione derivatives as PPAR-γ modulators. Arch Pharm (Weinheim). https://doi.org/10.1002/ARDP.201700223
Hinnah K, Willems S, Morstein J et al (2020) Photohormones enable optical control of the peroxisome proliferator-activated receptor γ (PPARγ). J Med Chem. https://doi.org/10.1021/acs.jmedchem.0c00654
Shakour N, Sahebkar A, Karimi G et al (2021) Design, synthesis and biological evaluation of novel 5-(imidazolyl-methyl) thiazolidinediones as antidiabetic agents. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105162
Feng L, Lu S, Zheng Z et al (2021) Identification of an allosteric hotspot for additive activation of PPARγ in antidiabetic effects. Sci Bull (Beijing) 66:1559–1570. https://doi.org/10.1016/j.scib.2021.01.023
Bar M, Skóra B, Tabęcka-Łonczyńska A et al (2022) New 4-thiazolidinone-based molecules Les-2769 and Les-3266 as possible PPARγ modulators. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2022.106075
Sun J, Liu HY, Zhang YH et al (2021) Design, synthesis and bioactivity evaluation of thiazolidinedione derivatives as partial agonists targeting PPARγ. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105342
Kumar AP, Mandal S, Prabitha P et al (2022) Rational design, molecular docking, dynamic simulation, synthesis, PPAR-γ competitive binding and transcription analysis of novel glitazones. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.133354
Mandal S, Chiriki DS, Gurupadayya BM et al (2022) Phenyl glycine incorporated glitazones as promising novel antidiabetic agents through PPARγ agonism: design, synthesis and preclinical studies. Eur J Med Chem Rep. https://doi.org/10.1016/j.ejmcr.2022.100067
Amin S, Sheikh KA, Iqubal A et al (2023) Synthesis, in-Silico studies and biological evaluation of pyrimidine based thiazolidinedione derivatives as potential anti-diabetic agent. Bioorg Chem. https://doi.org/10.1016/J.BIOORG.2023.106449
Kumar P, Duhan M, Kadyan K et al (2017) Synthesis of novel inhibitors of α-amylase based on the thiazolidine-4-one skeleton containing a pyrazole moiety and their configurational studies. Medchemcomm 8:1468–1476. https://doi.org/10.1039/C7MD00080D
Teng H, Chen L (2017) α-Glucosidase and α-amylase inhibitors from seed oil: a review of liposoluble substance to treat diabetes. Crit Rev Food Sci Nutr 57:3438–3448. https://doi.org/10.1080/10408398.2015.1129309
Qin X, Ren L, Yang X et al (2011) Structures of human pancreatic α-amylase in complex with acarviostatins: implications for drug design against type II diabetes. J Struct Biol 174:196–202. https://doi.org/10.1016/J.JSB.2010.11.020
de Sales PM, de Souza PM, Simeoni LA et al (2012) α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci 15:141–183. https://doi.org/10.18433/J35S3K
Bashary R, Vyas M, Nayak SK et al (2020) An insight of alpha-amylase inhibitors as a valuable tool in the management of type 2 diabetes mellitus. Curr Diabetes Rev 16:117–136. https://doi.org/10.2174/1573399815666190618093315
Rathod CH, Nariya PB, Maliwal D et al (2021) Design, synthesis and antidiabetic activity of biphenylcarbonitrile-thiazolidinedione conjugates as potential α-amylase inhibitors. ChemistrySelect 6:2464–2469. https://doi.org/10.1002/SLCT.202004362
Yousuf S, Khan KM, Salar U et al (2018) 2’-Aryl and 4’-arylidene substituted pyrazolones: as potential α-amylase inhibitors. Eur J Med Chem 159:47–58. https://doi.org/10.1016/J.EJMECH.2018.09.052
Naeem F, Nadeem H, Muhammad A et al (2018) Synthesis, α-Amylase Inhibitory Activity and Molecular Docking Studies of 2,4-Thiazolidinedione Derivatives. Open Chem J 5:134–144. https://doi.org/10.2174/1874842201805010134
Addanki HR, Vallabhaneni MR, Chennamsetty S et al (2022) An in silico ADMET, molecular docking study and microwave-assisted synthesis of new phosphorylated derivatives of thiazolidinedione as potential anti-diabetic agents. Synth Commun 52:300–315. https://doi.org/10.1080/00397911.2021.2024574
Doddagaddavalli MA, Kalalbandi VKA, Seetharamappa J (2023) Synthesis, characterization, crystallographic, binding, in silico and antidiabetic studies of novel 2,4-thiazolidinedione-phenothiazine molecular hybrids. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.134625
Gupta S, Baweja GS, Gupta GD, Asati V (2023) Identification of potential N-substituted 5-benzylidenethiazolidine-2,4-dione derivatives as α-amylase inhibitors: computational cum synthetic studies. J Mol Struct. https://doi.org/10.1016/j.molstruc.2023.135596
Singh R, Kumar P, Sindhu J et al (2023) Parsing structural fragments of thiazolidin-4-one based α-amylase inhibitors: A combined approach employing in vitro colorimetric screening and GA-MLR based QSAR modelling supported by molecular docking, molecular dynamics simulation and ADMET studies. Comput Biol Med. https://doi.org/10.1016/j.compbiomed.2023.106776
Kaur R, Kumar R, Dogra N, Yadav AK (2022) Design, synthesis, biological evaluations and in silico studies of sulfonate ester derivatives of 2-(2-benzylidenehydrazono)thiazolidin-4-one as potential α-glucosidase inhibitors. J Mol Struct 1247:131266. https://doi.org/10.1016/J.MOLSTRUC.2021.131266
Jiang B, Luo J, Guo S, Wang L (2021) Discovery of 5-(3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzylidene)thiazolidine-2,4-dione as a novel potent protein tyrosine phosphatase 1B inhibitor with antidiabetic properties. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.104648
Thareja S, Verma SK, Jain AK et al (2023) Rational design and synthesis of novel biphenyl thiazolidinedione conjugates as inhibitors of protein tyrosine phosphatase 1B for the management of type 2 diabetes. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.134546
Mohd Siddique MU, Thakur A, Shilkar D et al (2021) Non-carboxylic acid inhibitors of aldose reductase based on N-substituted thiazolidinedione derivatives. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2021.113630
Kumar Pasala V, Gudipudi G, Sankeshi V et al (2021) Design, synthesis and biological evaluation of selective hybrid coumarin-thiazolidinedione aldose reductase-II inhibitors as potential antidiabetics. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.104970
Kratky M, Sramel P, Bodo P et al (2023) Novel rhodanine based inhibitors of aldose reductase of non-acidic nature with p-hydroxybenzylidene functional group. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2022.114922
Thari FZ, Fettach S, Anouar EH et al (2022) Synthesis, crystal structures, α-glucosidase and α-amylase inhibition, DFT and molecular docking investigations of two thiazolidine-2,4-dione derivatives. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.132960
Gummidi L, Kerru N, Ebenezer O et al (2021) Multicomponent reaction for the synthesis of new 1,3,4-thiadiazole-thiazolidine-4-one molecular hybrids as promising antidiabetic agents through α-glucosidase and α-amylase inhibition. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105210
Angajala G, Aruna V, Pavan P, Guruprasad Reddy P (2022) Biocatalytic one pot three component approach: Facile synthesis, characterization, molecular modelling and hypoglycemic studies of new thiazolidinedione festooned quinoline analogues catalyzed by alkaline protease from Aspergillus niger. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2021.105533
Shah M, Jan MS, Sadiq A et al (2023) SAR and lead optimization of (Z)-5-(4-hydroxy-3-methoxybenzylidene)-3-(2-morpholinoacetyl)thiazolidine-2,4-dione as a potential multi-target antidiabetic agent. Eur J Med Chem 258:115591. https://doi.org/10.1016/j.ejmech.2023.115591
Khan AA, Ullah H, Rahim F et al (2023) Synthesis, in vitro α-glucosidase and α-amylase activities, and an in silico molecular docking study of triazinoindole-thiazolidinone hybrid derivatives. Chem Data Collect. https://doi.org/10.1016/j.cdc.2023.101035
Acknowledgements
The authors are thankful to Guru Nanak Dev University for providing them the basic facilities to do this work. NK acknowledges funds from ICMR-BMI (BMI/11(04)/2022) for completing this work.
Funding
Nitish Kumar,ICMR-BMI (BMI/11(04)/2022)
Author information
Authors and Affiliations
Contributions
AS contributed toward conceptualization, roles/writing—original draft, data curation, and writing—review & editing; NK contributed toward investigation, validation, funding acquisition, formal analysis, and writing—review & editing; HKG, RR, AK, J, and MD contributed toward writing—review & editing; JVS and PSB contributed toward project administration, formal analysis, and Supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A., Kumar, N., Gulati, H.K. et al. Antidiabetic potential of thiazolidinedione derivatives with efficient design, molecular docking, structural activity relationship, and biological activity: an update review (2021–2023). Mol Divers (2024). https://doi.org/10.1007/s11030-023-10793-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10793-6