Abstract
Heterocyclic compounds are attractive candidates because of their vast applications in natural and physical sciences. Thienothiophene (TT) is an annulated ring of two thiophene rings with a stable and electron-rich structure. Thienothiophenes (TTs) fully represent the planar system, which can drastically alter or improve the fundamental properties of organic, π-conjugated materials when included into a molecular architecture. These molecules possessed many applications including, pharmaceutical as well as optoelectronic properties. Different isomeric forms of thienothiophene showed various applications such as antiviral, antitumor, antiglaucoma, antimicrobial, and as semiconductors, solar cells, organic field effect transistors, electroluminiscents etc. A number of methodologies were adopted to synthesize thienothiophene derivatives. In this review, we have addressed different synthetic strategies of various isomeric forms of thienothiophene that have been reported during last seven years, i.e., 2016–2022.
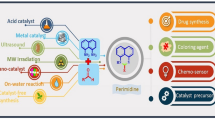
Graphical abstract






































Similar content being viewed by others
Abbreviations
- Al2O3 :
-
Aluminium oxide
- Br-TT-PhCN:
-
4-(2,5-Dibromothieno[3,2-b]thiophen-3-yl) benzonitrile
- (CH2)CN2 :
-
Malononitrile
- CS2 :
-
Carbon disulphide
- DCM:
-
Dichloromethane
- DDQ:
-
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
- DMF:
-
Dimethyl formamide
- DMSO:
-
Dimethyl sulphoxide
- LED:
-
Light-emiting diode
- Mes2BF:
-
Dimesitylboron flouride
- n-BuLi:
-
n-Butyl Lithium
- NBS:
-
N-Bromosuccinimide
- NEt3 :
-
Triethylamine
- OFETs:
-
Organic field effect transistors
- OLEDs:
-
Organic light emitting diodes
- Pd2(dba)3 :
-
Tris(dibenzylideneacetone)dipallium (0)
- Pd(OAc)2 :
-
Palladium (II) acetate
- P(o-tolyl)3 :
-
Tris(o-tolyl)phosphine
- PPA:
-
Polyphosphoric acid
- Pd (PPh3)4 :
-
Tetrakis(triphenylphosphine)Palladium
- Pd(PPh3)2Cl2 :
-
Bis(Triphenylphosphine)palladium (II) dichloride
- p-TsOH:
-
p-Toluenesulfonic acid
- TEA:
-
Triethylamine
- THF:
-
Tetrahydrofuran
- TPA:
-
Triphenylaminme
- TPE:
-
Tetraphenylethylene
- TPE-TTs:
-
Tetraphenylethylene-thienothiophenes
- TT:
-
Thienothiophene
- TT-TPA:
-
Thienothiophene-triphenylamine
- TT-TPA-TPE3:
-
Thienothiophene-tripheneylamine-tetraphenylethylene
- TTs:
-
Thienothiophenes
References
Thakral S, Singh V (2019) Recent development on importance of heterocyclic amides as potential bioactive molecules: a review. Curr Bioact Compd 15:316–336. https://doi.org/10.2174/1573407214666180614121140
Iftikhar R, Khan FZ, Naeem N (2023) Recent synthetic strategies of small heterocyclic organic molecules with optoelectronic applications : a review. Mol Divers. https://doi.org/10.1007/s11030-022-10597-0
Mabkhoot YN (2009) Synthesis and analysis of some bis-heterocyclic compounds containing sulphur. Molecules 14:1904–1914. https://doi.org/10.3390/molecules14051904
Mishra R, Jha KK, Kumar S, Tomer I (2011) Synthesis, properties and biological activity of thiophene: a review. Der Pharma Chemica 3:38–54
Wu C, Decker ER, Blok N, Bui H, You TJ, Wang J, Bourgoyne AR, Knowles V, Berens KL, Holland GW, Brock TA, Dixon RAF (2004) Discovery, modeling, and human pharmacokinetics of N-(2-acetyl-4,6-dimethylphenyl)-3-(3,4-dimethylisoxazol-5-ylsulfamoyl) -thiophene-2-carboxamide (TBC3711), a second generation, ETA selective, and orally bioavailable endothelin antagonist. J Med Chem 47:1969–1986. https://doi.org/10.1021/jm030528p
Podlesný J, Bureš F (2022) Thienothiophene scaffolds as building blocks for (opto)electronics. Organics 3:446–469. https://doi.org/10.3390/org3040029
Leriche P, Raimundo JM, Turbiez M, Monroche V, Allain M, Sauvage FX, Roncali J, Frére P, Skabara PJ (2003) Linearly extended tetrathiafulvalene analogues with fused thiophene units as π-conjugated spacers. J Mater Chem 13:1324–1332. https://doi.org/10.1039/b301149f
Mashraqui SH, Sangvikar Y, Ashraf M, Kumar S, Dâub ETH (2005) Dipyridyl/pyridiniumthieno[2,3-b]thiophenes as new atropisomeric systems. synthesis, conformational analysis and energy minimization. Tetrahedron 61:3507–3513. https://doi.org/10.1016/j.tet.2005.01.123
Mashraqui SH, Sangvikar YS, Meetsma A (2006) Synthesis and structures of thieno[2,3-b]thiophene incorporated [3.3]dithiacyclophanes. Enhanced first hyperpolarizability in an unsymmetrically polarized cyclophane. Tetrahedron Lett 47:5599–5602. https://doi.org/10.1016/j.tetlet.2006.05.098
Heeney M, Bailey C, Genevicius K, Shkunov M, Sparrowe D, Tierney S, McCulloch I (2005) Stable polythiophene semiconductors incorporating thieno[2,3-b]thiophene. J Am Chem Soc 127:1078–1079. https://doi.org/10.1021/ja043112p
Lee B, Seshadri V, Palko H, Sotzing GA (2005) Ring-sulfonated poly(thienothiophene). Adv Mater 17:1792–1795. https://doi.org/10.1002/adma.200500210
Kim HS, Kim YH, Kim TH, Noh YY, Pyo S, Yi MH, Kim DY, Kwon SK (2007) Synthesis and studies on 2-hexylthieno[3,2-b]thiophene end-capped oligomers for OTFTs. Chem Mater 19:3561–3567. https://doi.org/10.1021/cm070053g
Lim E, Jung BJ, Lee J, Shim HK, Lee JI, Yang YS, Do LM (2005) Thin-film morphologies and solution-processable field-effect transistor behavior of a fluorene - thieno[3,2-b]thiophene-based conjugated copolymer. Macromolecules 38:4531–4535. https://doi.org/10.1021/ma048128e
Jarak I, Kralj M, Piantanida I, Šuman L, Žinić M, Pavelić K, Karminski-Zamola G (2006) Novel cyano- and amidino-substituted derivatives of thieno[2,3-b]- and thieno[3,2-b]thiophene-2-carboxanilides and thieno[3′,2′:4,5]thieno- and thieno[2′,3′:4,5]thieno [2,3-c]quinolones: synthesis, photochemical synthesis, DNA binding, and antitumor evaluation. Bioorg Med Chem 14:2859–2868. https://doi.org/10.1016/j.bmc.2005.12.004
Cinar ME, Ozturk T (2015) Thienothiophenes, dithienothiophenes, and thienoacenes : syntheses, oligomers, polymers, and properties. Chem Rev 115:3036–3140. https://doi.org/10.1021/cr500271a
Comel A, Sommen G, Kirsch G (2005) Thienothiophenes: synthesis and applications. Mini-Rev Org Chem 1:367–374. https://doi.org/10.2174/1570193043403118
Kim J, Shim HS, Lee H, Choi MS, Kim JJ, Seo Y (2014) Highly efficient vacuum-processed organic solar cells containing thieno[3,2-b]thiophene-thiazole. J Phys Chem C 118:11559–11565. https://doi.org/10.1021/jp5017467
Bronstein H, Chen Z, Ashraf RS, Zhang W, Du J, Durrant JR, Tuladhar PS, Song K, Watkins SE, Wienk GY, MM, Janseen RAJ, Anthopolous T, Sirringhaus H, Heeney M, McCulloch I, (2011) Thieno[3,2-b]thiophene-diketopyrrolopyrrole containing polymers for high performance organic field effect transistors and organic photovoltaic devices. J Am Chem Soc 133:3272–3275. https://doi.org/10.1021/ja110619k
Chen YC, Chou HH, Tsai MC, Chen SY, Lin JT, Yao CF, Chen K (2012) Thieno[3,4-b]thiophene-based organic dyes for dye-sensitized solar cells. Chem Eur J 18:5430–5437. https://doi.org/10.1002/chem.201200012
Mabkhot YN, Barakat A, Al-Majid AM, Choudhary MI (2013) Synthesis of thieno[2,3-b]thiophene containing bis-heterocycles-novel pharmacophores. Int J Mol Sci 14:5712–5722. https://doi.org/10.3390/ijms14035712
Mabkhot YN, Kheder NA, Farag AM (2013) Synthesis and antimicrobial activity of some new thieno[2,3-b]thiophene derivatives. Molecules 18:4669–4678. https://doi.org/10.3390/molecules18044669
Výprachtický D, Demirtas I, Dzhabarov V, Pokorná V, Ertas E, Ozturk T, Cimrová V (2017) New copolymers with thieno[3,2-b]thiophene or dithieno [3,2-b:2’,3’-d]thiophene units possessing electron-withdrawing 4-cyanophenyl groups : synthesis and photophysical, electrochemical, and electroluminescent properties. J Polym Sci, Part A: Polym Chem 55:2629–2638. https://doi.org/10.1002/pola.28657
Isci R, Varzeghani AR, Kaya K, Sütay B, Tekin E, Ozturk T (2022) Triphenylamine/Tetraphenylethylene substituted 4-thieno[3,2-b]thiophen-3-ylbenzonitriles: synthesis, photophysical-electronic properties, and applications. ACS Sustain Chem Eng 10:1605–1615. https://doi.org/10.1021/acssuschemeng.1c07240
Ilhan KT, Topal S, Eroglu MS, Ozturk T (2019) Concise synthesis of 3-alkylthieno[3,2-b]thiophene; building block for organic electronic and optoelectronic materials. RSC Adv 9:38407–38413. https://doi.org/10.1039/c9ra08023f
Topal S, Topal S, Ulukan P, Ustamehmetoğlu B, Ozturk T, Sezer E (2021) Synthesis and characterization of 3-(4-fluorophenyl) thieno [3,2-b] thiophene and 3, 3′-(4-fluorophenyl) dithieno [3,2-b;2′,3′-d] thiophene molecules. Electrochim Acta 390:138837. https://doi.org/10.1016/j.electacta.2021.138837
Gunturkun D, Isci R, Sütay B, Majewski LA, Faraji S, Ozturk T (2022) Copolymers of 3-arylthieno [3,2-b] thiophenes bearing different substituents : synthesis, electronic, optical, sensor and memory properties. Eur Polym J 170:111167. https://doi.org/10.1016/j.eurpolymj.2022.111167
Isci R, Tekin E, Mucur SP, Ozturk T (2020) A bifunctional bulky thienothiophene derivative; synthesis, electronic-optical properties and OLED applications. ChemistrySelect 5:13091–13098. https://doi.org/10.1002/slct.202003273
Turkoglu G, Cinar ME, Buyruk A, Tekin E, Mucur SP, Kaya K, Ozturk T (2016) Novel organoboron compounds derived from thieno[3,2-b] thiophene and triphenylamine units for OLED devices. J Mater Chem C 4:6045–6053. https://doi.org/10.1039/c6tc01285j
Isci R, Tekin E, Kaya K, Mucur SP, Gorkem SF, Ozturk T (2020) Tetraphenylethylene substituted thienothiophene and dithienothiophene derivatives: synthesis, optical properties and OLED applications. J Mater Chem C 8:7908–7915. https://doi.org/10.1039/d0tc01715a
Cinar ME, Engür E, Ozturk T (2018) Synthesis and characterization of organic light-emitting molecules possessing 3-(4-methoxyphenyl) thieno [3,2-b] thiophene and boron. Org Commun 11:68–74. https://doi.org/10.25135/acg.oc.45.18.05.103
Manuela M, Raposo M, Herbivo C, Hugues V, Clermont G, Castro MCR, Comel A, Blanchard-Desce M (2016) Synthesis, fluorescence, and two-photon absorption properties of push–pull 5-arylthieno[3,2-b]thiophene derivatives. Eur J Org Chem 2016:5263–5273. https://doi.org/10.1002/ejoc.201600806
Fernandes SS, Castro MCR, Mesquita I, Andrade L, Mendes A, Raposo MMM (2016) Synthesis and characterization of novel thieno[3,2-b]thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells. Dyes Pigm 136:46–53. https://doi.org/10.1016/j.dyepig.2016.08.020
Fernandes SS, Mesquita I, Andrade L, Mendes A, Justino LL, Burrows HD, Raposo MMM (2017) Synthesis and characterization of push-pull bithiophene and thieno[3,2-b]thiophene derivatives bearing an ethyne linker as sensitizers for dye-sensitized solar cells. Org Electron 49:194–205. https://doi.org/10.1016/j.orgel.2017.06.048
Sarjadi MS, Iraqi A (2016) Synthesis of carbazole based co-polymers containing thienothiophene and benzothiadiazole units in a direct arylation scheme. Polym Polym Compos 24:703–710. https://doi.org/10.1177/096739111602400905
Buyruk A, Cinar ME, Eroglu MS, Ozturk T (2016) Polymerization of thienothiophenes and dithienothiophenes via Click-reaction for electronic applications. ChemistrySelect 1:3028–3032. https://doi.org/10.1002/slct.201600697
Li Y, Lee TH, Park SY, Uddin MA, Kim T, Hwang S, Kim JY, Woo HY (2016) Straight chain D-A copolymers based on thienothiophene and benzothiadiazole for efficient polymer field effect transistors and photovoltaic cells. Polym Chem 7:4638–4646. https://doi.org/10.1039/c6py00674d
Kim MJ, Choi JY, An G, Kim H, Kang Y, Kim JK, Son HJ, Lee JH, Cho JH, Kim B (2016) A new rigid planar low band gap PTTDPP-DT-DTT polymer for organic transistors and performance improvement through the use of a binary solvent system. Dyes Pigm 126:138–146. https://doi.org/10.1016/j.dyepig.2015.11.022
Turkoglu G, Cinar ME, Ozturk T (2017) Synthesis and photophysical and anion sensing properties of triarylborane-substituted cross-conjugated and conjugated thienothiophenes. Eur J Org Chem 2017:4552–4561. https://doi.org/10.1002/ejoc.201700679
Madathil PK, Cho S, Choi S, Kim TD, Lee KS (2018) Synthesis and characterization of cyclopentadithiophene and thienothiophene-based polymers for organic thin-film transistors and solar cells. Macromol Res 26:934–941. https://doi.org/10.1007/s13233-018-6130-0
Le TH, Dao QD, Nghiém MP, Péralta S, Guillot R, Pham QN, Fujii A, Ozaki M, Goubard F, Bui TT (2018) Triphenylamine – thienothiophene organic charge-transport molecular materials : effect of substitution pattern on their thermal, photoelectrochemical, and photovoltaic properties. Chem An Asian J 13:1302–1311. https://doi.org/10.1002/asia.201701790
Zhai W, Tang A, Xiao B, Wang X, Chen F, Zhou E (2018) A small molecular electron acceptor based on asymmetric hexacyclic core of thieno[1,2-b]indaceno[5,6-b′]thienothiophene for efficient fullerene-free polymer solar cells. Sci Bull 63:845–852. https://doi.org/10.1016/j.scib.2018.05.025
Sarjadi MS, Tan SE, Iraqi A (2018) Preparation and properties of fluorene-based thienothiophene substituted benzothiadiazole copolymer for the application of polymeric solar cells. Malays J Chem 20:24–32
Podlesný J, Pytela O, Klikar M, Jelínková V, Kityk IV, Ozga K, Jedryka J, Rudysh M, Bureš F (2019) Small isomeric push-pull chromophores based on thienothiophenes with tunable optical (non)linearities. Org Biomol Chem 17:3623–3634. https://doi.org/10.1039/c9ob00487d
Marco AB, Martínez de Baroja N, Andrés-Castán JM, Franco S, Andreu R, Villacampa B, Orduna J, Garín J (2019) Pyranylidene/thienothiophene-based organic sensitizers for dye-sensitized solar cells. Dyes Pigm 161:205–213. https://doi.org/10.1016/j.dyepig.2018.09.035
Jo Y, Oh GJ, Kim C, An TK, Jang J, Lee J (2020) Synthetic strategy for thienothiophene-benzotriazole-based polymers with high backbone planarity and solubility for field-effect transistor applications. J Ind Eng Chem 86:150–157. https://doi.org/10.1016/j.jiec.2020.02.022
Sousa RPCL, Gonçalves RCR, Costa SPG, Figueira RB, Raposo MMM (2021) Heterocyclic aldehydes based on thieno[3,2-b] thiophene Core : Synthesis and preliminary studies as ion optical chemosensors. Chem proced 3:88. https://doi.org/10.3390/ecsoc-24-08092
Vasilev A, Kostadinov A, Kandinska M, Landfester K, Baluschev S (2022) Tetrathienothiophene porphyrin as a metal-free sensitizer for room-temperature triplet – triplet annihilation up conversion. Front Chem 10:809863. https://doi.org/10.3389/fchem.2022.809863
Talamo MM, Pop F, Hume P, Abbas M, Wantz G, Avarvari N (2022) Helical thienothiophene (TT) and benzothieno-benzothiophene (BTBT) derivatives: synthesis, structural characterization and semiconducting properties. J Mater Chem C 10:8034–8042. https://doi.org/10.1039/d2tc00861k
Rahman MM, Alamry KA, Awual MR, Mekky AEM (2020) Efficient Hg (II) ionic probe development based on one-step synthesized diethyl thieno [2,3-b] thiophene-2,5-dicarboxylate (DETTDC2) onto glassy carbon electrode. Microchem J 152:104291. https://doi.org/10.1016/j.microc.2019.104291
Wang D, Wan YP, Liu H, Wang DJ, Yin GD (2018) Synthesis, photoluminescent and electrochemical properties of diacetoxyboron derivatives for bis- β -diketone linked thienothiophene. Dyes Pigm 149:728–735. https://doi.org/10.1016/j.dyepig.2017.11.030
Mohamed MAA, Salah H, El-Saghier AMM (2019) Synthesis, reactions and biological evaluation of some novel thienothiophene derivatives. Eur J Chem 10:209–217. https://doi.org/10.5155/eurjchem.10.3.209-217.1850
Cinar ME, Cankaya ST, Capan A, Eroglu MS, Ozturk T (2018) Thieno[2,3-b] thiophene based polymers: synthesis and optoelectronic properties. Eur Polymer J 104:72–80. https://doi.org/10.1016/j.eurpolymj.2018.05.001
Aly KI, Moustafa AH, Ahmed EK, Abd El-Lateef HM, Mohamed MG, Mohamed SM (2018) New polymer syntheses part 60: a facile synthetic route to polyamides based on thieno[2,3-b]thiophene and their corrosion inhibition behavior. Chin J Polym Sci 36:835–847. https://doi.org/10.1007/s10118-018-2101-3
Ali TE, Assiri MA (2021) A convenient one-pot synthesis of novel functionalized thiophene, thieno[2,3-b] thiophene, thiopyran, and thiopyrano[2,3-b]thiopyran bearing phosphonate groups. J Sulfur Chem 42:490–498. https://doi.org/10.1080/17415993.2021.1909027
Gomha SM, Edrees MM, El-Arab EE (2016) Synthesis and preliminary In-Vitro cytotoxic evaluation of some novel bis-heterocycles incorporating thienothiophene. J Heterocycl Chem 54:641–647. https://doi.org/10.1002/jhet.2636
Qiu F, Yang J, Shi D, Zhang Q, Li J (2016) Synthesis of thieno[2,3-b]thiophene fused pyrimidine derivatives via sequential conversion of 3,4-diaminothieno[2,3-b]thiophene-2,5-dicarbonitrile with carbonyl compounds. Tetrahedron Lett 57:1210–1214. https://doi.org/10.1016/j.tetlet.2016.01.040
Gomha SM, Badrey MG, Edrees MM (2016) Heterocyclisation of 2,5-diacetyl-3,4-disubstituted-thieno[2,3-b] thiophene bis-thiosemicarbazones leading to bis-thiazoles and bis-1,3,4-thiadiazoles anti-breast cancer agents. J Chem Res 40:120–125. https://doi.org/10.3184/174751916x14537182696214
Sayed OM, Mekky AEM, Farag AM, Elwahy AHM (2016) 3,4-bis(bromomethyl)thieno[2,3-b]thiophene: versatile precursors for novel bis(triazolothiadiazines), bis(quinoxalines), bis(dihydrooxadiazoles), and bis(dihydrothiadiazoles). J Heterocycl Chem 53:1113–1120. https://doi.org/10.1002/jhet.2373
Gomha SM, El-Hashash MA, Edrees MM, El-Arab EE (2017) Synthesis, characterization, and molecular docking of novel bis-thiazolyl thienothiophene derivatives as promising cytotoxic antitumor drug. J Heterocycl Chem 54:2686–2695. https://doi.org/10.1002/jhet.2869
Eid EM, Hassaneen HME, Abdelhamid IA, Elwahy AHM (2020) Facile one-pot, three-component synthesis of novel bis( heterocycles) incorporating thieno [2,3-b] thiophenes via Michael addition reaction. J Heterocycl Chem 57:2243–2255. https://doi.org/10.1002/jhet.3945
El-Remailya MAAA, Shokr EK, Abdel-Ghany H, Kamel MS, Elhady OM, Soliman AM (2022) Synthesis of some new thieno[2,3-b ]thiophene derivatives and prediction their biological activity by PASS INET. Sohag J Sc 7:9–13. https://doi.org/10.21608/sjsci.2022.233419
Chagas GR, Darmanin T, Godeau G, Guittard F (2018) Nanocups and hollow microspheres formed by a one-step and templateless electropolymerization of thieno [3,4-b]thiophene derivatives as a function of the substituent. Electrochim Acta 269:462–478. https://doi.org/10.1016/j.electacta.2018.03.036
Chao YC, Chen JH, Chiou YJ, Kao PL, Wu JL, Chen CT, Chan LH, Jeng RJ (2020) Design of thienothiophene-based copolymers with various side chain-end groups for efficient polymer solar cells. Polymers 12:2964. https://doi.org/10.3390/polym12122964
Author information
Authors and Affiliations
Contributions
Ayesha Rafiq, Sana Aslam, Muhammad Shahid Nazir and Ambar Farooq conducted the thorough literature survey and wrote the primary draft. Matloob Ahmad and Sadia Sultan updated the manuscript to its final form. Matloob Ahmad supervised the activity.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rafiq, A., Aslam, S., Ahmad, M. et al. Recent synthetic approaches towards thienothiophenes: a potential template for biologically active compounds. Mol Divers (2023). https://doi.org/10.1007/s11030-023-10647-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10647-1