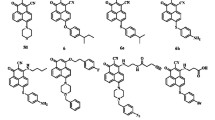
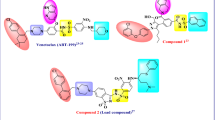
Abstract
Elevated expression of anti-apoptotic proteins, such as Bcl-2 and Mcl-1 contributes to poor prognosis and resistance to current treatment modalities in multiple cancers. Here, we report the design, synthesis and characterization of benzimidazole chalcone and flavonoid scaffold-derived bicyclic compounds targeting both Bcl-2 and Mcl-1 by optimizing the structural differences in the binding sites of both these proteins. Initial docking screen of Bcl-2 and Mcl-1 with pro-apoptotic protein Bim revealed possible hits with optimal binding energies. All the optimized bicyclic compounds were screened for their in vitro cytotoxic activity against two oral cancer cell lines (AW8507 and AW13516) which express high levels of Bcl-2 and Mcl-1. Compound 4d from the benzimidazole chalcone series and compound 6d from the flavonoid series exhibited significant cytotoxic activity (IC50 7.12 μM and 17.18 μM, respectively) against AW13516 cell line. Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) analysis further demonstrated that compound 4d and compound 6d could effectively inhibit the Bcl-2 and Mcl-1 proteins by displacing their BH3 binding partners. Both compounds exhibited potent activation of canonical pathway of apoptosis evident from appearance of cleaved Caspase-3 and PARP. Further, treatment of oral cancer cells with the inhibitors induced dissociation of the BH3 only protein Bim from Mcl-1 and Bak from Bcl-2 but failed to release Bax from Bcl-xL thereby confirming the nature of compounds as BH3-mimetics selectively targeting Bcl-2 and Mcl-1. Our study thus identifies bicyclic compounds as promising candidates for anti-apoptotic Bcl-2/Mcl-1 dual inhibitors with a potential for further development.
Graphical abstract









Similar content being viewed by others
References
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. https://doi.org/10.1016/S0092-8674(00)81683-9
Delbridge ARD, Strasser A (2015) The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ 22:1071–1080. https://doi.org/10.1038/cdd.2015.50
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337. https://doi.org/10.1038/sj.onc.1210220
Chipuk JE, Green DR (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18:157–164. https://doi.org/10.1016/j.tcb.2008.01.007
Akgul C (2009) Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci 66:1326–1336. https://doi.org/10.1007/s00018-008-8637-6
Mallick S, Patil R, Gyanchandani R et al (2009) Human oral cancers have altered expression of Bcl-2 family members and increased expression of the anti-apoptotic splice variant of Mcl-1. J Pathol 217:398–407. https://doi.org/10.1002/path.2459
Shahar N, Larisch S (2020) Inhibiting the inhibitors: targeting anti-apoptotic proteins in cancer and therapy resistance. Drug Resist Updat 52:100712. https://doi.org/10.1016/j.drup.2020.100712
Singh R, Letai A, Sarosiek K (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20:175–193. https://doi.org/10.1038/s41580-018-0089-8
Warren CFA, Wong-Brown MW, Bowden NA (2019) BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis 10:177. https://doi.org/10.1038/s41419-019-1407-6
Ertel F, Nguyen M, Roulston A, Shore GC (2013) Programming cancer cells for high expression levels of Mcl1. EMBO Rep 14:328–336. https://doi.org/10.1038/embor.2013.20
Fletcher S (2019) MCL-1 inhibitors—where are we now (2019)? Expert Opin Ther Pat 29:909–919. https://doi.org/10.1080/13543776.2019.1672661
Kang MH, Reynolds CP (2009) Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 15:1126–1132. https://doi.org/10.1158/1078-0432.CCR-08-0144
Negi A, Murphy PV (2021) Development of Mcl-1 inhibitors for cancer therapy. Eur J Med Chem 210:113038. https://doi.org/10.1016/j.ejmech.2020.113038
Prakesch M, Denisov AY, Naim M et al (2008) The discovery of small molecule chemical probes of Bcl-XL and Mcl-1. Bioorg Med Chem 16:7443–7449. https://doi.org/10.1016/j.bmc.2008.06.023
Lessene G, Czabotar PE, Colman PM (2008) BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov 7:989–1000. https://doi.org/10.1038/nrd2658
van Delft MF, Wei AH, Mason KD et al (2006) The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 10:389–399. https://doi.org/10.1016/j.ccr.2006.08.027
Tse C, Shoemaker AR, Adickes J et al (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421–3428. https://doi.org/10.1158/0008-5472.CAN-07-5836
Kaefer A, Yang J, Noertersheuser P et al (2014) Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother Pharmacol 74:593–602. https://doi.org/10.1007/s00280-014-2530-9
Mason KD, Carpinelli MR, Fletcher JI et al (2007) Programmed anuclear cell death delimits platelet life span. Cell 128:1173–1186. https://doi.org/10.1016/j.cell.2007.01.037
Negi A, Voisin‐Chiret AS (2022) Strategies to reduce the on‐target platelet toxicity of Bcl‐xL inhibitors: PROTACs, SNIPERs and prodrug‐based approaches. ChemBioChem. https://doi.org/10.1002/cbic.202100689
Souers AJ, Leverson JD, Boghaert ER et al (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19:202–208. https://doi.org/10.1038/nm.3048
Nguyen M, Marcellus RC, Roulston A et al (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci 104:19512–19517. https://doi.org/10.1073/pnas.0709443104
De Blasio A, Vento R, Di Fiore R (2018) Mcl-1 targeting could be an intriguing perspective to cure cancer. J Cell Physiol 233:8482–8498. https://doi.org/10.1002/jcp.26786
Konopleva M, Contractor R, Tsao T et al (2006) Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10:375–388. https://doi.org/10.1016/j.ccr.2006.10.006
Sulkshane P, Pawar SN, Waghole R et al (2021) Elevated USP9X drives early-to-late-stage oral tumorigenesis via stabilisation of anti-apoptotic MCL-1 protein and impacts outcome in oral cancers. Br J Cancer 125:547–560. https://doi.org/10.1038/s41416-021-01421-x
Wood KC (2020) Overcoming MCL-1-driven adaptive resistance to targeted therapies. Nat Commun 11:531. https://doi.org/10.1038/s41467-020-14392-z
Wei Y, Cao Y, Sun R et al (2020) Targeting Bcl-2 proteins in acute myeloid leukemia. Front Oncol 10:584974. https://doi.org/10.3389/fonc.2020.584974
Grundy M, Balakrishnan S, Fox M et al (2018) Genetic biomarkers predict response to dual BCL-2 and MCL-1 targeting in acute myeloid leukaemia cells. Oncotarget 9:37777–37789. https://doi.org/10.18632/oncotarget.26540
Carter BZ, Mak PY, Tao W et al (2020) Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and re-sensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica 107:58–76. https://doi.org/10.3324/haematol.2020.260331
Liu F, Zhao Q, Su Y et al (2022) Cotargeting of Bcl-2 and Mcl-1 shows promising antileukemic activity against AML cells including those with acquired cytarabine resistance. Exp Hematol 105:39–49. https://doi.org/10.1016/j.exphem.2021.10.006
Belmar J, Fesik SW (2015) Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacol Ther 145:76–84. https://doi.org/10.1016/j.pharmthera.2014.08.003
Lamie PF, Philoppes JN (2021) Design, synthesis, stereochemical determination, molecular docking study, in silico pre-ADMET prediction and anti-proliferative activities of indole-pyrimidine derivatives as Mcl-1 inhibitors. Bioorgan Chem 116:105335. https://doi.org/10.1016/j.bioorg.2021.105335
Hird AW, Tron AE (2019) Recent advances in the development of Mcl-1 inhibitors for cancer therapy. Pharmacol Ther 198:59–67. https://doi.org/10.1016/j.pharmthera.2019.02.007
Wang H, Guo M, Wei H, Chen Y (2021) Targeting MCL-1 in cancer: current status and perspectives. J Hematol OncolJ Hematol Oncol 14:67. https://doi.org/10.1186/s13045-021-01079-1
Goard CA, Schimmer A (2013) An evidence-based review of obatoclax mesylate in the treatment of hematological malignancies. Core Evid. https://doi.org/10.2147/CE.S42568
Sulkshane P, Teni T (2017) BH3 mimetic Obatoclax (GX15–070) mediates mitochondrial stress predominantly via MCL-1 inhibition and induces autophagy-dependent necroptosis in human oral cancer cells. Oncotarget 8:60060–60079. https://doi.org/10.18632/oncotarget.11085
Voss V, Senft C, Lang V et al (2010) The Pan-Bcl-2 inhibitor (−)-gossypol triggers autophagic cell death in malignant glioma. Mol Cancer Res 8:1002–1016. https://doi.org/10.1158/1541-7786.MCR-09-0562
Friberg A, Vigil D, Zhao B et al (2013) Discovery of potent myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods and structure-based design. J Med Chem 56:15–30. https://doi.org/10.1021/jm301448p
Tatake RJ, Rajaram N, Damle RN et al (1990) Establishment and characterization of four new squamous cell carcinoma cell lines derived from oral tumors. J Cancer Res Clin Oncol 116:179–186. https://doi.org/10.1007/BF01612674
Anantram A, Kundaikar H, Degani M, Prabhu A (2019) Molecular dynamic simulations on an inhibitor of anti-apoptotic Bcl-2 proteins for insights into its interaction mechanism for anti-cancer activity. J Biomol Struct Dyn 37:3109–3121. https://doi.org/10.1080/07391102.2018.1508371
Adams JM, Cory S (2018) The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ 25:27–36. https://doi.org/10.1038/cdd.2017.161
Zhong D, Gu C, Shi L et al (2014) Obatoclax induces G1/G0-phase arrest via p38/p21waf1/Cip1 signaling pathway in human esophageal cancer cells. J Cell Biochem 115:1624–1635. https://doi.org/10.1002/jcb.24829
Zinkel S, Gross A, Yang E (2006) BCL2 family in DNA damage and cell cycle control. Cell Death Differ 13:1351–1359. https://doi.org/10.1038/sj.cdd.4401987
Widden H, Placzek WJ (2021) The multiple mechanisms of MCL1 in the regulation of cell fate. Commun Biol 4:1029. https://doi.org/10.1038/s42003-021-02564-6
Bonnefoy-Berard N, Aouacheria A, Verschelde C et al (2004) Control of proliferation by Bcl-2 family members. Biochim Biophys Acta BBA - Mol Cell Res 1644:159–168. https://doi.org/10.1016/j.bbamcr.2003.10.014
Du X, Fu X, Yao K et al (2017) Bcl-2 delays cell cycle through mitochondrial ATP and ROS. Cell Cycle 16:707–713. https://doi.org/10.1080/15384101.2017.1295182
Du X, Xiao J, Fu X et al (2021) A proteomic analysis of Bcl-2 regulation of cell cycle arrest: insight into the mechanisms. J Zhejiang Univ-Sci B 22:839–855. https://doi.org/10.1631/jzus.B2000802
Jamil S, Sobouti R, Hojabrpour P et al (2005) A proteolytic fragment of Mcl-1 exhibits nuclear localization and regulates cell growth by interaction with Cdk1. Biochem J 387:659–667. https://doi.org/10.1042/BJ20041596
Moujalled DM, Pomilio G, Ghiurau C et al (2019) Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia 33:905–917. https://doi.org/10.1038/s41375-018-0261-3
Song T, Wang Z, Ji F et al (2016) Deactivation of Mcl-1 by Dual-function small-molecule inhibitors targeting the Bcl-2 homology 3 domain and facilitating Mcl-1 ubiquitination. Angew Chem Int Ed 55:14250–14256. https://doi.org/10.1002/anie.201606543
Algarín EM, Díaz-Tejedor A, Mogollón P et al (2020) Preclinical evaluation of the simultaneous inhibition of MCL-1 and BCL-2 with the combination of S63845 and venetoclax in multiple myeloma. Haematologica 105:e116–e120. https://doi.org/10.3324/haematol.2018.212308
Moujalled DM, Hanna DT, Hediyeh-zadeh S et al (2020) Cotargeting BCL-2 and MCL-1 in high-risk B-ALL. Blood Adv 4:2762–2767. https://doi.org/10.1182/bloodadvances.2019001416
Glantz-Gashai Y, Meirson T, Reuveni E, Samson AO (2017) Virtual screening for potential inhibitors of Mcl-1 conformations sampled by normal modes, molecular dynamics, and nuclear magnetic resonance. Drug Des Devel Ther 11:1803–1813. https://doi.org/10.2147/DDDT.S133127
Cao H, Sethumadhavan K, Cao F, Wang TTY (2021) Gossypol decreased cell viability and down-regulated the expression of a number of genes in human colon cancer cells. Sci Rep 11:5922. https://doi.org/10.1038/s41598-021-84970-8
Ahmed NM, Youns MM, Soltan MK, Said AM (2021) Design, synthesis, molecular modeling and antitumor evaluation of novel indolyl-pyrimidine derivatives with EGFR inhibitory activity. Molecules 26:1838. https://doi.org/10.3390/molecules26071838
Abou Samra A, Robert A, Gov C et al (2018) Dual inhibitors of the pro-survival proteins Bcl-2 and Mcl-1 derived from natural compound meiogynin A. Eur J Med Chem 148:26–38. https://doi.org/10.1016/j.ejmech.2018.01.100
Zhang Z, Song T, Zhang T et al (2011) A novel BH3 mimetic S1 potently induces Bax/Bak-dependent apoptosis by targeting both Bcl-2 and Mcl-1. Int J Cancer 128:1724–1735. https://doi.org/10.1002/ijc.25484
Zhu J, Wang Z, Guo Z et al (2020) Structure-based design, synthesis, and evaluation of Bcl-2/Mcl-1 dual inhibitors. Arch Pharm. https://doi.org/10.1002/ardp.202000005
Xu G, Liu T, Zhou Y et al (2017) 1-Phenyl-1H-indole derivatives as a new class of Bcl-2/Mcl-1 dual inhibitors: design, synthesis, and preliminary biological evaluation. Bioorgan Med Chem 25:5548–5556. https://doi.org/10.1016/j.bmc.2017.08.024
Acknowledgements
The authors would like to thank the following core facilities for their contributions to this work: Drug design and synthesis experiments were performed in the Institute of Chemical Technology, Department of Pharmaceutical Sciences and Technology; NMR and Computer Aided Drug Design Facility for instrumentation. This facility receives support from TEQIP Grant (TEQIP II/TEQIP-II/MH/MH2G01/386/347).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing financial or non-financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uthale, A., Anantram, A., Sulkshane, P. et al. Identification of bicyclic compounds that act as dual inhibitors of Bcl-2 and Mcl-1. Mol Divers 27, 1359–1374 (2023). https://doi.org/10.1007/s11030-022-10494-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10494-6