Abstract
In order to find novel environment-friendly and effective antifungal agents, four series of 2,2-dimethyl-2H-chromene derivatives were designed, synthesized and characterized by spectroscopic analysis. The antifungal activities of all the target compounds against nine phytopathogenic fungi were evaluated in vitro. Preliminary results indicated that most of the target compounds exhibited obvious antifungal activity at the concentration of 50 μg/mL. Among them, compound 4j displayed more promising antifungal potency against Fusarium solani, Pyricularia oryzae, Alternaria brassicae, Valsa mali and Alternaria alternata strains than the two commercially available fungicides chlorothalonil and hymexazol, with the corresponding EC50 values of 6.3, 7.7, 7.1, 7.5, 4.0 μg/mL, respectively. Moreover, the cell experiments results suggested that the target compounds had low cytotoxicity to the PC12 cell. This research will provide theoretical basis for the future application of 2,2-dimethyl-2H-chromenes as botanical fungicides in agriculture.
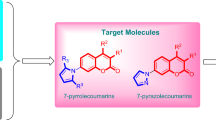
Graphical Abstract

Four series of novel, potent and low-toxicity 2,2-dimethyl-2H-chromene derivatives were designed and synthesized as agricultural antifungal agents. The in vitro antifungal experiments showed that compound 4j exhibited higher antifungal efficacy against five strains than the two commercially-available fungicides chlorothalonil and hymexazol.










Similar content being viewed by others
References
Ons L, Bylemans D, Thevissen K, Cammue BPA (2020) Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 8(12):1930. https://doi.org/10.3390/microorganisms8121930
Brauer VS, Rezende CP, Pessoni AM, Paula RG, Rangappa SK, Nayaka SC, Gupta VK, Almeida F (2019) Antifungal agents in agriculture: friends and foes of public health. Biomolecules 9:521. https://doi.org/10.3390/biom9100521
Sparks TC, Bryant RJ (2021) Impact of natural products on discovery of, and innovation in, crop protection compounds. Pest Manag Sci. https://doi.org/10.1002/ps.6653
Agarwal SK, Verma S, Singh SS, Tripathi AK, Khan ZK, Kumar S (2000) Antifeedant and antifungal activity of chromene compounds isolated from Blepharispermum subsessile. J Ethnopharmacol 71(1–2):231–234. https://doi.org/10.1016/S0378-8741(00)00158-6
Meepagala KM, Schrader KK, Burandt CL, Wedge DE, Duke SO (2010) New class of algicidal compounds and fungicidal activities derived from a chromene amide of Amyris texana. J Agr Food Chem 58(17):9476–9482. https://doi.org/10.1021/jf101626g
Saga Kitamura RO, Romoff P, Young MCM, Kato MJ, Lago JHG (2006) Chromenes from Peperomia serpens (Sw.) Loudon (Piperaceae). Phytochemistry 67(21):2398–2402. https://doi.org/10.1016/j.phytochem.2006.08.007
Malquichagua Salazar KJ, Delgado Paredes GE, Lluncor LR, Young MCM, Kato MJ (2005) Chromenes of polyketide origin from Peperomia villipetiola. Phytochemistry 66(5):573–579. https://doi.org/10.1016/j.phytochem.2005.01.003
Yadav N, Ganie SA, Singh B, Chhillar AK, Yadav SS (2019) Phytochemical constituents and ethnopharmacological properties of Ageratum conyzoides L. Phytother Res 33(9):2163–2178. https://doi.org/10.1002/ptr.6405
Zheng GW, Luo SH, Li SF, Hua J, Li WQ, Li SH (2018) Specialized metabolites from Ageratina adenophora and their inhibitory activities against pathogenic fungi. Phytochemistry 148:57–62. https://doi.org/10.1016/j.phytochem.2018.01.013
Pratap R, Ram VJ (2014) Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem Rev 114(20):10476–10526. https://doi.org/10.1021/cr500075s
Simmler C, Pauli GF, Chen SN (2013) Phytochemistry and biological properties of glabridin. Fitoterapia 90:160–184. https://doi.org/10.1016/j.fitote.2013.07.003
Lee JW, Choe SS, Jang H, Kim J, Jeong HW, Jeong KH, Tadi S, Park MG, Kwak TH, Kim JM, Hyun DH, Bum Kim JB (2012) AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J Lipid Res 53:1277–1286. https://doi.org/10.1194/jlr.m022897
Pratt GE, Jennings RC, Hamnett AF, Brooks GT (1980) Lethal metabolism of precocene-I to a reactive epoxide by locust Corpora allata. Nature 284:320–323. https://doi.org/10.1038/284320a0
Mao LX, Henderson G, Dong SL (2010) Studies of precocene I, precocene II and biogenic amines on formosan subterranean Termite (Isoptera: Rhinotermitidae) presoldier/soldier formation. J Entomol Sci 45(1):27–34. https://doi.org/10.18474/0749-8004-45.1.27
Samnesh ET, Tayebeh R, Hassan E, Vahid N (2010) Composition of essential oils in subterranean organs of three species of Valeriana L. Nat Prod Res 24:1834–1842. https://doi.org/10.1080/14786419.2010.482051
Parra JE, Delgado WA, Cuca LE (2011) Cumanensic acid, a new chromene isolated from Piper cf. cumanense Kunth. (Piperaceae). Phytochem Lett 4(3):280–282. https://doi.org/10.1016/j.phytol.2011.04.015
Abrunhosa L, Costa M, Areias F, Venâncio A, Proença F (2007) Antifungal activity of a novel chromene dimer. J Ind Microbiol Biot 34(12):787–792. https://doi.org/10.1007/s10295-007-0255-z
Xu H, Fan LL (2011) Synthesis and antifungal activities of novel 5,6-dihydroindolo-[1,2-a]quinoxaline derivatives. Eur J Med Chem 46(5):1919–1925. https://doi.org/10.1016/j.ejmech.2011.02.035
Xu H, Fan LL (2011) Antifungal agents. Part 4: synthesis and antifungal activities of novel indole[1,2-c]-1,2,4-benzotriazine derivatives against phytopathogenic fungi in vitro. Eur J Med Chem 46(1):364–369. https://doi.org/10.1016/j.ejmech.2010.10.022
Fan LL, Luo BL, Luo ZF, Zhang L, Fan JD, Xue W, Tang L, Li Y (2019) Synthesis and antifungal activities of 3-substituted phthalide derivatives. Z Naturforsch B 74(11–12):811–818. https://doi.org/10.1515/znb-2019-0110
Li Y, Luo ZF, Luo BL, Lan Q, Fan JD, Xue W, Miao J, Li Y, Tang L, Fan LL (2020) Design, synthesis and antifungal activities of 6-substituted 3-butylphthalide derivatives against phytopathogenic fungi. Chem Biodivers 17:e2000435. https://doi.org/10.1002/cbdv.202000435
Fan LL, Luo ZF, Li Y, Liu XY, Fan JD, Xue W, Tang L, Li Y (2020) Synthesis and antifungal activity of imidazo[1,2-b]pyridazine derivatives against phytopathogenic fungi. Bioorg Med Chem Lett 30:127139. https://doi.org/10.1016/j.bmcl.2020.127139
Luo ZF, Deng Y, Luo BL, Li Y, Lan Q, Fan JD, Xue W, Tang L, Fan LL (2021) Design and synthesis of novel n-butyphthalide derivatives as promising botanical fungicides. Z Naturforsch C 76(3–4):117–127. https://doi.org/10.1515/znc-2020-0192
Fan LL, Luo ZF, Yang CF, Tang L, Li Y (2021) Design and synthesis of small molecular 2-aminobenzoxazoles as potential antifungal agents against phytopathogenic fungi. Mol Divers. https://doi.org/10.1007/s11030-021-10213-7
Yang CP, Xie LJ, Ma YQ, Cai XW, Yue GZ, Qin GG, Zhang M, Gong GS, Chang XL, Qiu XY, Luo LY, Chen HB (2021) Study on the fungicidal mechanism of glabridin against Fusarium graminearum. Pestici Biochem Phys 179:104963. https://doi.org/10.1016/j.pestbp.2021.104963
López-López E, Bajorath J, Medina-Franco JL (2021) Informatics for chemistry, biology, and biomedical Sciences. J Chem Inf Model 61(1):26–35. https://doi.org/10.1021/acs.jcim.0c01301
Ishikawa T, Mizutani A, Miwa C, Oku Y, Komano N, Takami A, Watanabe T (1997) Cesium fluoride-mediated claisen rearrangements of phenyl propargyl ethers: substituent effects of an ortho-alkoxy group on the benzene ring or modified propargyl residues. Heterocycles 45(11):2261–2272. https://doi.org/10.3987/com-97-7946
Soussi MA, Audisio D, Messaoudi S, Provot O, Brion JD, Alami M (2011) Palladium-catalyzed coupling of 3-halo-substituted coumarins, chromenes, and quinolones with various nitrogen-containing nucleophiles. Eur J Org Chem 26:5077–5088. https://doi.org/10.1002/ejoc.201100480
Ji WH, Gao QS, Lin YL, Gao HM, Wang X, Geng YL (2014) Total synthesis of (±)-glabridin. Synth Commun 44(4):540–546. https://doi.org/10.1080/00397911.2013.821616
Salvatore MM, Andolfi A (2021) Phytopathogenic fungi and toxicity. Toxins 13(10):689. https://doi.org/10.3390/toxins13100689
Wu W, Zhou J, Pan XX, Pan Q, Shi L, Zang LQ (2009) Experimental study on primary rat cortical neurons and PC12 cells used for the evaluation of acute cytotoxicity. J Clin Res 26(3):369–373. https://doi.org/10.3969/j.issn.1671-7171.2009.03.001
Fournier L, Aujard I, Saux TL, Maurin S, Beaupierre S, Baudin JB, Jullien L (2013) Coumarinylmethyl caging groups with redshifted absorption. Chem Eur J 19(51):17494–17507. https://doi.org/10.1002/chem.201302630
Chen L, Hu TS, Yao ZJ (2008) Development of new pyrrolocoumarin derivatives with satisfactory fluorescent properties and notably large stokes shifts. Eur J Org Chem 36:6175–6182. https://doi.org/10.1002/ejoc.200800883
Acknowledgements
This research was supported by The National Natural Science Foundation of China (No.32060627). Guizhou Provincial Natural Science Foundation (No. [2020]1Y111), The State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering/Key Laboratory of Ministry of Education, Guizhou University (No. 2021GDP0101, QJHKYZ[2022]362 and QJHKYZ[2020]250), The Project of Postgraduate Scientific Research Fund of Guizhou (No. YJSKYJJ [2021] 152).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Luo, B., Luo, Z. et al. Design and synthesis of novel 2,2-dimethylchromene derivatives as potential antifungal agents. Mol Divers 27, 589–601 (2023). https://doi.org/10.1007/s11030-022-10421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10421-9