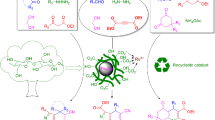
Abstract
Novel sulfonated carbon-coated magnetic nanoparticles (SCCMNPs; Fe3O4@C@OSO3H) were designed, synthesized, characterized, and applied as an efficient nanocatalyst for green synthesis of coumarin derivatives through Pechmann condensation. The Fe3O4@C@OSO3H was manufactured through a simple and inexpensive two-step procedure and characterized by FTIR, EDX, XRD, SEM, TEM, DLS, VSM, and TGA techniques. It was identified as an efficient heterogeneous catalyst in the Pechmann condensation of phenol derivatives and β-ketoesters, leading to high-yield coumarin derivatives under solvent-free conditions. The Fe3O4@C@OSO3H removed after reaction finishing point by an external magnet, and it was reused fifteen times at the same conditions. Besides, theoretical studies were carried out using B3LYP/6-311++G(d,p) to more consideration of the reaction mechanism. The study of the frontier molecular orbitals, NBO atomic charges, molecular electrostatic potential of reactants, as well as Pechmann condensation mechanism was known very useful in suitable reactant choice. The reaction was performed through the electrophilic attack, dehydration, and trans-esterification, respectively.
Graphic abstract


















Similar content being viewed by others
References
Ogoshi T, Kanai S, Fujinami S, Yamagishi T-A, Nakamoto Y (2008) para-bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host-guest property. J Am Chem Soc 130(15):5022–5023
Han Z-Y, Xiao H, Chen X-H, Gong L-Z (2009) Consecutive intramolecular hydroamination/asymmetric transfer hydrogenation under relay catalysis of an achiral gold complex/chiral Brønsted acid binary system. J Am Chem Soc 131(26):9182–9183
Muratore ME et al (2009) Enantioselective Brønsted acid-catalyzed N-acyliminium cyclization cascades. J Am Chem Soc 131(31):10796–10797
Lee YH et al (2016) Removal of benzoic acid in heavy oils by esterification using modified ferrierite: roles of Brønsted and Lewis acid sites. Energy Fuels 30(7):5391–5397
Clark JH (2002) Solid acids for green chemistry. Acc Chem Res 35(9):791–797
Anastas PT, Kirchhoff MM (2002) Origins, current status, and future challenges of green chemistry. Acc Chem Res 35(9):686–694
Smith K, El-Hiti GA, Jayne AJ, Butters M (2003) Acetylation of aromatic ethers using acetic anhydride over solid acid catalysts in a solvent-free system. Scope of the reaction for substituted ethers. Org Biomol Chem 1(9):1560–1564
Hara M et al (2004) A carbon material as a strong protonic acid. Angew Chem Int Ed 43(22):2955–2958
Melero JA, Bautista LF, Morales G, Iglesias J, Briones D (2009) Biodiesel production with heterogeneous sulfonic acid-functionalized mesostructured catalysts. Energy Fuels 23(1):539–547
Morales G, Paniagua M, Melero JA, Vicente G, Ochoa C (2011) Sulfonic acid-functionalized catalysts for the valorization of glycerol via transesterification with methyl acetate. Ind Eng Chem Res 50(10):5898–5906
Zareyee D, Moosavi SM, Alaminezhad A (2013) Chemoselective synthesis of geminal diacetates (acylals) using eco-friendly reusable propylsulfonic acid based nanosilica (SBA-15-Ph-PrSO3H) under solvent-free conditions. J Mol Catal A-Chem 378:227–231
Nakajima K, Hara M (2012) Amorphous carbon with SO3H groups as a solid Brønsted acid catalyst. ACS Catal 2(7):1296–1304
Fukuhara K et al (2011) Structure and catalysis of cellulose-derived amorphous carbon bearing SO3H groups. Chemsuschem 4(6):778–784
Akiyama G, Matsuda R, Sato H, Takata M, Kitagawa S (2011) Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv Mater 23(29):3294–3297
Lu A-H, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46(8):1222–1244
Kim J et al (2006) Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J Am Chem Soc 128(3):688–689
Abbasi Z, Rezayati S, Bagheri M, Hajinasiri R (2017) Preparation of a novel, efficient, and recyclable magnetic catalyst, γ-Fe2O3@HAp-Ag nanoparticles, and a solvent- and halogen-free protocol for the synthesis of coumarin derivatives. Chin Chem Lett 28(1):75–82
Ghavidel H, Mirza B, & Soleimani-Amiri S (2019) A Novel, efficient, and recoverable basic Fe3O4@C nano-catalyst for green synthesis of 4H-chromenes in water via one-pot three component reactions. Polycyclic Aromat Compd. https://doi.org/10.1080/10406638.2019.1607413
Soleimani-Amiri S, Arabkhazaeli M, Hossaini Z, Afrashteh S, Eslami AA (2018) Synthesis of chromene derivatives via three-component reaction of 4-hydroxycumarin catalyzed by magnetic Fe3O4 nanoparticles in water. J Heterocycl Chem 55(1):209–213
Keshavarz M, Zarei Ahmady A, Vaccaro L, Kardani M (2018) Non-covalent supported of l-proline on graphene oxide/Fe3O4 nanocomposite: a novel, highly efficient and superparamagnetically separable catalyst for the synthesis of bis-pyrazole derivatives. Molecules 23(2):16
Ahmady AZ, Saghanezhad SJ, Mohtasham N (2017) Sulfuric acid functionalized magnetic nanocatalyst for one-pot green synthesis of 2,3-dihydroquinazolin-4 (1H)-ones. J Nanoanal 4(4):313–319
Zhang M, Liu Y-H, Shang Z-R, Hu H-C, Zhang Z-H (2017) Supported molybdenum on graphene oxide/Fe3O4: an efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal Commun 88:39–44
Chen M-N, Mo L-P, Cui Z-S, Zhang Z-H (2019) Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr Opin Green Sustain Chem 15:27–37
Zhang M et al (2016) Magnetically separable graphene oxide anchored sulfonic acid: a novel, highly efficient and recyclable catalyst for one-pot synthesis of 3,6-di(pyridin-3-yl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles in deep eutectic solvent under microwave irradiation. RSC Adv 6(108):106160–106170
Wang P, Kong A, Wang W, Zhu H, Shan Y (2010) Facile preparation of ionic liquid functionalized magnetic nano-solid acid catalysts for acetalization reaction. Catal Lett 135(1):159–164
Karimi B, Mirzaei HM, Mobaraki A, Vali H (2015) Sulfonic acid-functionalized periodic mesoporous organosilicas in esterification and selective acylation reactions. Catal Sci Technol 5(7):3624–3631
Elhamifar D, Ramazani Z, Norouzi M, Mirbagheri R (2018) Magnetic iron oxide/phenylsulfonic acid: a novel, efficient and recoverable nanocatalyst for green synthesis of tetrahydrobenzo[b]pyrans under ultrasonic conditions. J Colloid Interface Sci 511:392–401
Esfahani FK, Zareyee D, Yousefi R (2014) Sulfonated core–shell magnetic nanoparticle (Fe3O4@SiO2@PrSO3H) as a highly active and durable protonic acid catalyst; synthesis of coumarin derivatives through Pechmann reaction. ChemCatChem 6(12):3333–3337
Dumitrache F et al (2004) Nearly monodispersed carbon coated iron nanoparticles for the catalytic growth of nanotubes/nanofibres. Diamond Relat Mater 13(2):362–370
Zheng J et al (2012) One-step solvothermal synthesis of Fe3O4@C core–shell nanoparticles with tunable sizes. Nanotechnol 23(16):165601
Polshettiwar V et al (2011) Magnetically recoverable nanocatalysts. Chem Rev 111(5):3036–3075
Naeimi H, Mohamadabadi S (2014) Sulfonic acid-functionalized silica-coated magnetic nanoparticles as an efficient reusable catalyst for the synthesis of 1-substituted 1H-tetrazoles under solvent-free conditions. Dalton Trans 43(34):12967–12973
Mahmoudi H, Jafari AA (2013) Facial preparation of sulfonic acid-functionalized magnetite-coated maghemite as a magnetically separable catalyst for pyrrole synthesis. ChemCatChem 5(12):3743–3749
Mobaraki A, Movassagh B, Karimi B (2014) Magnetic solid sulfonic acid decorated with hydrophobic regulators: a combinatorial and magnetically separable catalyst for the synthesis of α-aminonitriles. ACS Comb Sci 16(7):352–358
Samadizadeh M, Nouri S, Kiani Moghadam F (2016) Magnetic nanoparticles functionalized ethane sulfonic acid (MNESA): as an efficient catalyst in the synthesis of coumarin derivatives using Pechmann condensation under mild condition. Res Chem Intermed 42(6):6089–6103
Nasseri MA, Sadeghzadeh SM (2014) Diazabicyclo[2.2.2]octane stabilized on Fe3O4 as catalysts for synthesis of coumarin under solvent-free conditions. J Iran Chem Soc 11(1):27–33
Fan G-j et al (2001) A novel class of inhibitors for steroid 5α-reductase: synthesis and evaluation of umbelliferone derivatives. Bioorg Med Chem Lett 11(17):2361–2363
Wang C-J, Hsieh Y-J, Chu C-Y, Lin Y-L, Tseng T-H (2002) Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett 183(2):163–168
Spino C, Dodier M, Sotheeswaran S (1998) Anti-HIV coumarins from Calophyllum seed oil. Bioorg Med Chem Lett 8(24):3475–3478
Ghosh PP, Das AR (2013) Nanocrystalline and reusable ZnO catalyst for the assembly of densely functionalized 4H-chromenes in aqueous medium via one-pot three component reactions: a greener, “NOSE” approach. J Org Chem 78(12):6170–6181
Cravotto G, Nano GM, Palmisano G, Tagliapietra S (2001) An asymmetric approach to coumarin anticoagulants via hetero-Diels–Alder cycloaddition. Tetrahedron Asymmetry 12(5):707–709
Sharghi H, Jokar M (2007) Al2O3/MeSO3H (AMA) as a novel heterogeneous system for synthesis of coumarins under mild conditions. Heterocycles 71(12):2721–2733
Sharma G, Reddy JJ, Lakshmi PS, Krishna PR (2005) An efficient ZrCl4 catalyzed one-pot solvent free protocol for the synthesis of 4-substituted coumarins. Tetrahedron Lett 46(36):6119–6121
Gu Y, Zhang J, Duan Z, Deng Y (2005) Pechmann reaction in non-chloroaluminate acidic ionic liquids under solvent-free conditions. Adv Synth Catal 347(4):512–516
Zareyee D, Serehneh M (2014) Recyclable CMK-5 supported sulfonic acid as an environmentally benign catalyst for solvent-free one-pot construction of coumarin through Pechmann condensation. J Mol Catal A: Chem l 391:88–91
Prousis KC, Avlonitis N, Heropoulos GA, Calogeropoulou T (2014) FeCl3-catalysed ultrasonic-assisted, solvent-free synthesis of 4-substituted coumarins. A useful complement to the Pechmann reaction. Ultrason Sonochem 21(3):937–942
Jafari E, Farajzadeh P, Akbari N, Karbakhshzadeh A (2019) An efficient and facile synthesis of the coumarin and ester derivatives using sulfonated polyionic liquid as a highly active heterogeneous catalyst. Chem Rev Lett 2(3):123–129
Ghodke S, Chudasama U (2013) Solvent free synthesis of coumarins using environment friendly solid acid catalysts. Appl Catal A 453:219–226
Ahmed AI, El-Hakam S, Khder A, El-Yazeed WA (2013) Nanostructure sulfated tin oxide as an efficient catalyst for the preparation of 7-hydroxy-4-methyl coumarin by Pechmann condensation reaction. J Mol Catal A: Chem 366:99–108
Mokhtary M, Najafizadeh F (2012) Polyvinylpolypyrrolidone-bound boron trifluoride (PVPP-BF3); a mild and efficient catalyst for synthesis of 4-metyl coumarins via the Pechmann reaction. C R Chim 15(6):530–532
Bouasla S et al (2017) Coumarin derivatives solvent-free synthesis under microwave irradiation over heterogeneous solid catalysts. Molecules 22(12):2072
Tyagi B, Mishra MK, Jasra RV (2007) Synthesis of 7-substituted 4-methyl coumarins by Pechmann reaction using nano-crystalline sulfated-zirconia. J Mol Catal A: Chem 276(1–2):47–56
Daru J, Stirling A (2011) Mechanism of the Pechmann reaction: a theoretical study. J Org Chem 76(21):8749–8755
Sethna SM, Shah NM (1945) The chemistry of coumarins. Chem Rev 36(1):1–62
Torviso R et al (2008) Catalytic activity of Keggin heteropolycompounds in the Pechmann reaction. Appl Catal A 339(1):53–60
Calvino-Casilda V, Bañares M, LozanoDiz E (2010) Real-time Raman monitoring during coumarins synthesis via Pechmann condensation: a tool for controlling the preparation of pharmaceuticals. Catal Today 155(3–4):279–281
Tyndall S, Wong KF, VanAlstine-Parris MA (2015) Insight into the mechanism of the Pechmann condensation reaction using NMR. J Org Chem 80(18):8951–8953
Pornsatitworakul S et al (2017) The coumarin synthesis: a combined experimental and theoretical study. Monatsh Chem 148(7):1245–1250
Suh S-E, Chen S, Houk K, Chenoweth DM (2018) The mechanism of the triple aryne–tetrazine reaction cascade: theory and experiment. Chem Sci 9(39):7688–7693
Han L, Xu B, Liu T (2018) Mechanisms of the synthesis of trialkylsubstituted alkenylboronates from unactivated internal alkynes catalyzed by copper: a theoretical study. J Organomet Chem 864:154–159
Altass HM, Khder AERS (2018) Preparation, characterization of highly active recyclable zirconium and tin tungstate catalysts and their application in Pechmann condensation reaction. React Kinet Mech Catal 125(1):16
Aoudjit L, Halliche D, Bachari K, Saadi A, Cherifi O (2017) Nickel-containing mesoporous silicas as a catalyst for the Pechmann condensation reaction. Theor Exp Chem 53:10
Jadhav NH, Sakate SS, Rasal NK, Shinde, Pawar RA (2019) Heterogeneously catalyzed Pechmann condensation employing the tailored Zn0.925Ti0.075O NPs: synthesis of coumarin. ACS Omega 4(5):8522–8527
Mirosanloo A, Zareyee D, Khalilzadeh MA (2018) Recyclable cellulose nanocrystal supported Palladium nanoparticles as an efficient heterogeneous catalyst for the solvent-free synthesis of coumarin derivatives via von Pechmann condensation. Appl Organomet Chem 32(12):e4546
Pakdel S, Akhlaghinia B, Mohammadinezhad A (2019) Fe3O4@boehmite-NH2-CoII NPs: an environment friendly nanocatalyst for solvent free synthesis of coumarin derivatives through Pechmann condensation reaction. Chem Afr 2(3):10
Sun R, Gao Y, Ma Y, Yang G, Li Y (2017) SnCl4 grafted on silica gel: an efficient catalyst for solvent-free synthesis of coumarins via the Pechmann condensation. J Iran Chem Soc 14:5
Borah KJ, Borah R (2011) Poly(4-vinylpyridine)-supported sulfuric acid: an efficient solid acid catalyst for the synthesis of coumarin derivatives under solvent-free conditions. Monatsh Chem 142(12):1253–1257
Keri RS, Hosamani KM, Seetharama Reddy HR (2009) A solvent-free synthesis of coumarins using phosphotungstic acid as catalyst. Catal Lett 131(1):321–327
Reddy YT et al (2008) Ceric ammonium nitrate (CAN): an efficient catalyst for the coumarin synthesis via Pechmann condensation using conventional heating and microwave irradiation. Synth Commun 38(13):2082–2088
Raju BC et al (2010) α-Glucosidase inhibitory antihyperglycemic activity of substituted chromenone derivatives. Bioorg Med Chem 18(1):358–365
Gaussian 09 (2016) In: Frisch GWTMJ, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ, Gaussian (eds) Inc., Wallingford CT
Becke AD (1996) Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J Chem Phys 104(3):1040–1046
Hehre WJ, Warren J, Radom L, Schleyer PVR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York, p 548
Foresman JB, Frisch Æ (2015) Exploring chemistry with electronic structure methods, 3rd edn. Gaussian, Inc., Pittsburgh
Glendening ED et al (2013) NBO 6.0. Theoretical Chemistry Institute, University of Wisconsin, Madison
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samiei, Z., Soleimani-Amiri, S. & Azizi, Z. Fe3O4@C@OSO3H as an efficient, recyclable magnetic nanocatalyst in Pechmann condensation: green synthesis, characterization, and theoretical study. Mol Divers 25, 67–86 (2021). https://doi.org/10.1007/s11030-019-10025-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-10025-w