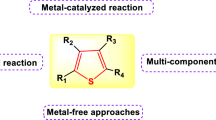
Abstract
Dihydropyrimidin-2(1H)-ones/thiones (DHPMs) are important heterocyclic compounds owing to their excellent biological activities and have been widely utilized in pharmaceutical applications. Recently, numerous DHPM derivatives have been prepared. This review covers the synthesis of DHPMs and improved procedures for the preparation of DHPMs from 1995 to 2016.



































































































































Similar content being viewed by others
References
Kappe CO (2000) Biologically active dihydropyrimidones of the Biginelli-type a literature survey. Eur J Med Chem 35:1043–1052. https://doi.org/10.1016/S02235234(00)01189-2
Snider BB, Chen J, Patil AD, Freyer A (1996) Synthesis of the tricyclic portions of batzelladines A, B and D. Revision of the stereoehemistry of batzelladines A and D. Tetrahedron Lett 37:6977–6980. https://doi.org/10.1016/0040-4039(96)01575-4
Kappe CO (2000) Recent advances in the Biginelli Dihydropyrimidine Synthesis. New Tricks from an old dog. Acc Chem Res 33: 879–888. https://doi.org/10.1021/ar000048h
Kumar A, Watar A, Maurya R (2007) Synthesis of 3,4-dihydropyrimidin-2(1H)-ones using Ziegler–Natta catalyst system under solvent free conditions. J Mol Catal A Chem 272:53–56. https://doi.org/10.1016/j.molcata.2007.03.026
Azizian J, Mohammadi AA, Karimi AR, Mohammadizadeh MR (2006) KAl\((\text{ SO }_{4})_{2}\cdot \text{12H }_{2}\text{ O }\) supported on silica gel as a novel heterogeneous system catalyzed biginelli reaction: one-pot synthesis of dihydropyrimidinones under solvent-free conditions. Appl Catal A Gen 300:85–88. https://doi.org/10.1016/j.apcata.2005.11.001
Dhumaskara KL, Meenab SN, Ghadi SC (2014) Graphite catalyzed solvent free synthesis of dihydropyrimidin-2(1H)-ones/thiones and their antidiabetic activity. Bioorg Med Chem Lett 24:2897–2899. https://doi.org/10.1016/j.bmcl.2014.04.099
Adibi H, Samimi HL, Beygzadeh M (2007) Iron(III) trifluoroacetate and trifluoromethanesulfonate: recyclable Lewis acid catalysts for one-pot synthesis of 3,4-dihydropyrimidinones or their sulfur analogues and 1,4-dihydropyridines via solvent-free Biginelli and Hantzsch condensation protocols. Catal Commun 8:2119–2124. https://doi.org/10.1016/j.catcom.2007.04.022
Velpula R, Banothu J, Gali R, Deshineni R, Bavantul R (2015) 1-Sulfopyridinium chloride: green and expeditious ionic liquid for the one-pot synthesis of fused 3,4-dihydropyrimidin-2(\(1H\))-ones and thiones under solvent-free conditions. Chin Chem Lett 26:309–312. https://doi.org/10.1016/j.cclet.2014.11.030
Karami B, Khodabakhshi S, Akrami S, Farahi M (2014) Regiospecific strategies for the synthesis of novel dihydropyrimidinones and pyrimidopyridazines catalyzed by molybdate sulfuric acid. Tetrahedron Lett 55:3581–3584. https://doi.org/10.1016/j.tetlet.2014.02.025
Lal J, Gupta SK, Thavaselvam D, Agarwal DD (2012) Design, synthesis, synergistic antimicrobial activity and cytotoxicity of 4-aryl substituted 3,4-dihydropyrimidinones of curcumin. Bioorg Med Chem Lett 22:2872–2876. https://doi.org/10.1016/j.bmcl.2012.02.056
Lal J, Sharma M, Gupta S, Parashar P, Sahu P, Agarwal DD (2012) Hydrotalcite: a novel and reusable solid catalyst for one-pot synthesis of 3,4-dihydropyrimidinones and mechanistic study under solvent free conditions. J Mol Catal A Chem 352:31–37. https://doi.org/10.1016/j.molcata.2011.09.009
Rao GBD, Acharya BN, Kaushik MP (2013) An efficient synthesis of \(\upbeta \)-ketoesters via transesterification and its application in Biginelli reaction under solvent-free, catalyst-free conditions. Tetrahedron Lett 54:6644–6647. https://doi.org/10.1016/j.tetlet.2013.09.130
Singh K, Arora D, Singh S (2006) Dowex-promoted general synthesis of N,N \(^\prime \)-disubstituted-4-aryl-3,4-dihydropyrimidinones using a solvent-free Biginelli condensation protocol. Tetrahedron Lett 47:4205–4207. https://doi.org/10.1016/j.tetlet.2006.04.061
Debache A, Amimour M, Belfaitah A, Rhouati S, Carboni B (2008) A one-pot Biginelli synthesis of 3,4-dihydropyrimidin-2-(\(1H\))-ones/thiones catalyzed by triphenylphosphine as Lewis base. Tetrahedron Lett 49:6119–6121. https://doi.org/10.1016/j.tetlet.2008.08.016
Balan B, Bahulayan D (2014) A novel green synthesis of \(\upalpha /\upbeta \)-amino acid functionalized pyrimidinone peptidomimetics using triazole ligation through click-multi-component reactions. Tetrahedron Lett 55:227–231. https://doi.org/10.1016/j.tetlet.2013.11.002
Rafiee E, Shahbazi F (2006) One-pot synthesis of dihydropyrimidones using silica-supported heteropoly acid as an efficient and reusable catalyst: improved protocol conditions for the Biginelli reaction. J Mol Catal A Chem 250:57–61. https://doi.org/10.1016/j.molcata.2006.01.049
Kefayati H, Asghari F, Khanjanian R (2012) 1-Methylimidazolium hydrogen sulfate/chlorotrimethylsilane: an effective catalytic system for the synthesis of 3,4-dihydropyrimidin-2(\(1H\))-ones and hydroquinazoline-2,5-diones. J Mol Liq 172:147–151. https://doi.org/10.1016/j.molliq.2012.01.019
Kalbasi RJ, Massah AR, Daneshvarnejad B (2012) Preparation and characterization of bentonite/PS-\(\text{ SO }_{3}\)H nanocomposites as an efficient acid catalyst for the Biginelli reaction. Appl Clay Sci 55:1–9. https://doi.org/10.1016/j.clay.2011.05.015
Nasr-Esfahani M, Hoseini SJ, Mohammadi F (2011) \(\text{ Fe }_{3}\text{ O }_{4}\) nanoparticles as an efficient and magnetically recoverable catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Chin J Catal 32:1484–1489. https://doi.org/10.1016/S1872-2067(10)60263-X
Jiang C, You QD (2007) An efficient and solvent-free one-pot synthesis of dihydropyrimidinones under microwave irradiation. Chin Chem Lett 18:647–650. https://doi.org/10.1016/j.cclet.2007.04.002
Shobha D, Chari MA (2009) An efficient Biginelli one-pot synthesis of new benzoxazole-substituted dihydropyrimidinones and thiones catalysed by trifluoro acetic acid under solvent-free conditions. Chin Chem Lett 20:1059–1061. https://doi.org/10.1016/j.cclet.2009.03.024
Nandurkar NS, Bhanushali MJ, Bhor MD, Bhanage BD (2007) \(\text{ Y }(\text{ NO }_{3})_{3}\cdot \text{6H }_{2}\text{ O }\): a novel and reusable catalyst for one pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. J Mol Catal A Chem 271:14–17. https://doi.org/10.1016/j.molcata.2007.02.021
Tayebee R, Amini MM, Ghadamgahi M, Armaghan M (2013) \(\text{ H }_{5}\text{ PW }_{10}\text{ V }_{2}\text{ O }_{40}\)/Pip-SBA-15: a novel reusable organic-inorganic hybrid material as potent Lewis acid catalyst for one-pot solvent-free synthesis of 3,4-dihydropyrimidinones. J Mol Catal A Chem 366:266–274. https://doi.org/10.1016/j.molcata.2012.10.004
Kour G, Gupta M, Paul S, Rajnikant Gupta VK (2014) \(\text{ SiO }_{2}\)-\(\text{ CuCl }_{2}\): an efficient and recyclable heterogeneous catalyst for one-pot synthesis of 3,4-dihydropyrimidin-2(\(1H\))-ones. J Mol Catal A Chem 392:260–269. https://doi.org/10.1016/j.molcata.2014.05.022
Kouachi K, Lafaye G, Pronier S, Bennini L, Menad S (2014) Mo/\(\gamma \)-\(\text{ Al }_{2}\text{ O }_{3}\) catalysts for the Biginelli reaction. Effect of Mo loading. J Mol Catal A Chem 395:210–216. https://doi.org/10.1016/j.molcata.2014.08.025
Chaudhary GR, Bansal P, Mehta SK (2014) Recyclable CuS quantum dots as heterogeneous catalyst for Biginelli reaction under solvent free conditions. Chem Eng J243:217–224. https://doi.org/10.1016/j.cej.2014.01.012
Choudhary VR, Tillu VH, Narkhede VS, Borate HB, Wakharkar RD (2003) Microwave assisted solvent-free synthesis of dihydropyrimidinones by Biginelli reaction over Si-MCM-41 supported \(\text{ FeCl }_{3}\) catalyst. Catal Commun 4:449–453. https://doi.org/10.1016/S1566-7367(03)00111-0
Singh V, Sapehiyia V, Srivastava V, Kaur S (2006) \(\text{ ZrO }_{2}\)-pillared clay: an efficient catalyst for solventless synthesis of biologically active multifunctional dihydropyrimidinones. Catal Commun 7:571–578. https://doi.org/10.1016/j.catcom.2005.12.021
Bigdeli MA, Jafari S, Mahdavinia GH, Hazakhani H (2007) Trichloroisocyanuric acid, a new and efficient catalyst for the synthesis of dihydropyrimidinones. Catal Commun 8:1641–1644. https://doi.org/10.1016/j.catcom.2007.01.022
Kamal A, Krishnaji T, Azhar MA (2007) Copper(II) tetrafluoroborate as a mild and efficient catalyst for the one-pot synthesis of 3,4-dihydropyrimidin-2(\(1H\))-ones under solvent-free conditions. Catal Commun 8:1929–1933. https://doi.org/10.1016/j.catcom.2007.03.009
Salim SD, Akamanchi KG (2011) Sulfated tungstate: an alternative, eco-friendly catalyst for Biginelli reaction. Catal Commun 12:1153–1156. https://doi.org/10.1016/j.catcom.2011.02.018
Ramachandran V, Arumugasamy K, Singh SK, Edayadulla N, Ramesh P, Kamaraj S (2016) Synthesis, antibacterial studies, and molecular modeling studies of 3,4-dihydropyrimidinone compounds. J Chem Biol 9:31–40. https://doi.org/10.1007/s12154-015-0142-4
Saikia M, Bhuyan D, Saikia L (2015) Keggin type phosphotungstic acid encapsulated chromium (III) terephthalate metal organic framework as active catalyst for Biginelli condensation. Appl Catal A Gen 505:501–506. https://doi.org/10.1016/j.apcata.2015.05.021
Khabazzadeh H, Saidi K, Sheibani H (2008) Microwave-assisted synthesis of dihydropyrimidin-2\((1H)\)-ones using graphite supported lanthanum chloride as a mild and efficient catalyst. Bioorg Med Chem Lett 18:278–280. https://doi.org/10.1016/j.bmcl.2007.10.087
Ahn BJ, Gang MS, Chae K, Oh Y, Shin J, Chang W (2008) A microwave-assisted synthesis of 3,4-dihydro-pyrimidin-2-\((1H)\)-ones catalyzed by \(\text{ FeCl }_{3}\)-supported Nanopore Silica under solvent-free conditions. J Ind Eng Chem 14:401–405. https://doi.org/10.1016/j.jiec.2008.01.008
Wang JH, Zhang E, Tang GM, Wang YT, Cui YZ, Ng SW (2016) Novel bipyridinyl oxadiazole-based metal coordination complexes: high efficient and green synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones through the Biginelli reactions. J Solid State Chem 241:86–98. https://doi.org/10.1016/j.jssc.2016.05.009
Kumar PM, Kumar KS, Poreddy SR, Mohakhud PK, Mukkanti K, Pal M (2011) Biginelli reaction beyond three-component limit: synthesis of functionalized pyrimidinones via a one-pot Biginelli-Pd mediated C-C coupling strategy. Tetrahedron Lett 52:1187–1191. https://doi.org/10.1016/j.tetlet.2011.01.015
Zhan HW, Wang JX, Wang XT (2008) Solvent- and catalyst-free synthesis of dihydropyrimidinthiones in one-pot under focused microwave irradiation conditions. Chin Chem Lett 19:1183–1185. https://doi.org/10.1016/j.cclet.2008.06.039
Bigdeli MA, Gholami G, Sheikhhosseini E (2011) \(P\)-Dodecylbenzenesulfonic acid (DBSA), a Brønsted acid-surfactant catalyst for Biginelli reaction in water and under solvent free conditions. Chin Chem Lett 22:903–906. https://doi.org/10.1016/j.cclet.2010.12.030
Elhamifar D, Hosseinpoor F, Karimi B, Hajati S (2015) Ionic liquid-based ordered mesoporous organosilica-supported copper as a novel and efficient nanocatalyst for the one-pot synthesis of Biginelli products. Micropor Mesopor Mat 204:269–275. https://doi.org/10.1016/j.micromeso.2014.11.011
Chen W, Qin S, Jin J (2007) HBF4-catalyzed Biginelli reaction: one-pot synthesis of dihydropyrimidin-2(1H)-ones under solvent-free conditions. Catal Commun 8:123–126. https://doi.org/10.1016/j.catcom.2006.05.026
Kalita HR, Phukan P (2007) CuI as reusable catalyst for the Biginelli reaction. Catal Commun 8:179–182. https://doi.org/10.1016/j.catcom.2006.06.004
Kargar M, Hekmatshoar R, Mostashari A, Hashemi Z (2011) Efficient and greensynthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones/thiones using imidazol-1-yl-acetic acid as a novel, reusable and water-soluble organocatalyst. Catal Commun 15:123–126. https://doi.org/10.1016/j.catcom.2011.08.022
Tayebee R, Maleki B, Ghadamgahi M (2012) Ammonium dihydrogen phosphate catalyst for one-pot synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones. Chin J Catal 33:659–665. https://doi.org/10.1016/S1872-2067(11)60355-0
Barbosa FAR, Canto RFS, Saba S, Rafique J, Braga AL (2016) Synthesis and evaluation of dihydropyrimidinone-derived selenoesters as multi-targeted directed compounds against Alzheimer’s disease. Bioorg Med Chem 24:5762–5770. https://doi.org/10.1016/j.bmc.2016.09.031
Su W, Li J, Zheng Z, Shen Y (2005) One-pot synthesis of dihydropyrimidiones catalyzed by strontium(II) triflate under solvent-free conditions. Tetrahedron Lett 46:6037–6040. https://doi.org/10.1016/j.tetlet.2005.07.021
Ahmed N, van Lier JE (2007) \(\text{ TaBr }_{5}\)-catalyzed Biginelli reaction: one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under solvent-free conditions. Tetrahedron Lett 48:5407–5409. https://doi.org/10.1016/j.tetlet.2007.06.005
Liberto NA, Silva SP, Fátima AD, Fernandes SA (2013) \(\upbeta \)-Cyclodextrin-assisted synthesis of Biginelli adducts under solvent-free conditions. Tetrahedron 69:8245–8249. https://doi.org/10.1016/j.tet.2013.07.024
Banik BK, Reddy AT, DattaA Mukhopadhyay C (2007) Microwave-induced bismuth nitrate-catalyzed synthesis of dihydropyrimidones via Biginelli condensation under solventless conditions. Tetrahedron Lett 48:7392–7394. https://doi.org/10.1016/j.tetlet.2007.08.007
Polshettiwar V, Varma RS (2007) Biginelli reaction in aqueous medium: a greener and sustainable approach to substituted 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett 48:7343–7346. https://doi.org/10.1016/j.tetlet.2007.08.031
Zhang H, Zhou Z, Yao Z, Xu F, Shen Q (2009) Efficient synthesis of pyrimidinone derivatives by ytterbium chloride catalyzed Biginelli-type reaction under solvent-free conditions. Tetrahedron Lett 50:1622–1624. https://doi.org/10.1016/j.tetlet.2009.01.103
Ghosh BK, Hazra S, Ghosh NN (2016) Synthesis of Cu@CF@SBA15: a Versatile catalysts for (i) reduction of dyes, trifluralin, Synthesis of (ii) DHPMs by Biginelli reaction and (iii) 1,2,3-triazole derivatives by ‘Click reaction’. Catal Commun 80:44–48. https://doi.org/10.1016/j.catcom.2016.03.016
Shirini F, Abedini M, Pourhasan-Kisomi R (2014) \(N\)-Sulfonic acid poly(4-vinylpyridinium) chloride as a highly efficient and reusable catalyst for the Biginelli reaction. Chin Chem Lett 25:111–114. https://doi.org/10.1016/j.cclet.2013.09.005
Dondoni A, Massi A (2001) Parallel synthesis of dihydropyrimidinones using Yb(III)-resin and polymer-supported scavengers under solvent-free conditions. A green chemistry approach to the Biginelli reaction. Tetrahedron Lett 42:7975–7978. https://doi.org/10.1016/S0040-4039(01)01728-2
Sari O, Roy V, Métifiot M, Marchand C, Pommier Y, Bourg S, Bonnet P, Schinazi RF, Agrofoglio LA (2015) Synthesis of dihydropyrimidine \(\upalpha \),\(\gamma \)-diketobutanoic acid derivatives targeting HIV integrase. Eur J Med Chem 104:127–138. https://doi.org/10.1016/j.ejmech.2015.09.015
Bigi F, Carloni S, Frullanti B, Maggi R, Sartori G (1999) A revision of the Biginelli reaction under solid acid catalysis. Solvent-free synthesis of dihydropyrimidines over montmorillonite KSF. Tetrahedron Lett 40:3465–3468. https://doi.org/10.1016/S0040-4039(99)00424-4
Wu M, Yu J, Zhao W, Wu J, Cao S (2011) One-pot synthesis of difluoromethyl-containing dihydropyrimidinones catalyzed by \(\text{ Yb }(\text{ PFO })_{3}\) under solvent and dehydrating agent free conditions. J Fluorine Chem 132:155–159. https://doi.org/10.1016/j.jfluchem.2010.12.010
Gong K, Wang H, Wang S, Ren X (2015) \(\upbeta \)-Cyclodextrin-propyl sulfonic acid: a new and eco-friendly catalyst for one-pot multi-component synthesis of 3,4-dihydropyrimidones via Biginelli reaction. Tetrahedron 71:4830–4834. https://doi.org/10.1016/j.tet.2015.05.028
Shaabani A, Bazgir A, Teimouri F (2003) Ammonium chloride-catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions. Tetrahedron Lett 44:857–859. https://doi.org/10.1016/S0040-4039(02)02612-6
Reddy KR, Reddy CV, Mahesh M, Raju PVK, Reddy VVN (2003) New environmentally friendly solvent free synthesis of dihydropyrimidinones catalysed by \(N\)-butyl-\(N\),\(N\)-dimethyl-\(\upalpha \)-phenylethylammonium bromide. Tetrahedron Lett 44:8173–8175. https://doi.org/10.1016/j.tetlet.2003.09.030
Aswin K, Mansoor SS, Logaiya K, Sudhan PN, Ahmed RN (2014) Facile synthesis of 3,4-dihydropyrimidin-2(1H)-ones and—thiones and indeno[1,2-d]pyrimidines catalyzed by p-dodecylbenzenesulfonic acid. J Taibah Uni Sci 8:236–247. https://doi.org/10.1016/j.jtusci.2014.03.005
Dong F, Jun L, Xinli Z, Zhiwen Y, Zuliang L (2007) One-pot green procedure for Biginelli reaction catalyzed by novel task-specific room-temperature ionic liquids. J Mol Catal A Chem 274:208–211. https://doi.org/10.1016/j.molcata.2007.05.014
Safari J, Gandomi-Ravandi S (2014) A novel protocol for solvent-free synthesis of 4,6-diaryl-3,4-dihydropyrimidine-2(1H)-ones catalyzed by metal oxide-MWCNTs nanocomposites. J Mol Struct 1074:71–78. https://doi.org/10.1016/j.molstruc.2014.05.012
Niknam K, Hasaninejad A, Arman M (2010) Synthesis of some new bis-3,4-dihydropyrimidin-2(1H)-ones by using silica-supported tin chloride and titanium tetrachloride. Chin Chem Lett 21:399–402. https://doi.org/10.1016/j.cclet.2009.12.008
Zamani F, Izadi E (2013) Synthesis and characterization of sulfonated-phenylacetic acid coated \(\text{ Fe }_{3}\text{ O }_{4}\) nanoparticles as a novel acid magnetic catalyst for Biginelli reaction. Catal Commun 42:104–108. https://doi.org/10.1016/j.catcom.2013.08.006
Harikrishnan PS, Rajesh SM, Perumal S, Almansour AI (2013) A microwave-mediated catalyst- and solvent-free regioselective Biginelli reaction in the synthesis of highly functionalized novel tetrahydropyrimidines. Tetrahedron Lett 54:1076–1079. https://doi.org/10.1016/j.tetlet.2012.12.034
Keivanloo A, Mirzaee M, Bakherad M, Soozani A (2014) Boehmite nanoparticle catalyst for the one-pot multicomponent synthesis of 3,4-dihydropyrimidin-2-\((1H)\)-ones and thiones under solvent-free conditions. Chin J Catal 35:362–367. https://doi.org/10.1016/S1872-2067(12)60759-1
Kolvari E, Koukabi N, Armandpour O (2014) A simple and efficient synthesis of 3,4-dihydropyrimidin-2-\((1H)\)-ones via Biginelli reaction catalyzed by nanomagnetic-supported sulfonic acid. Tetrahedron 70:1383–1386. https://doi.org/10.1016/j.tet.2013.10.085
Ghasemi Z, Farshbaf Orafa F, PirouzmandM Zarrini G, Nikzad Kojanag B, Salehi R (2015) \(\text{ Zn }^{2+}\)/MCM-41 catalyzed Biginelli reaction of heteroaryl aldehydes and evaluation of the antimicrobial activity and cytotoxicity of the pyrimidone products. Tetrahedron Lett 56:6393–6396. https://doi.org/10.1016/j.tetlet.2015.09.143
Tamaddon F, Moradi S (2013) Controllable selectivity in Biginelli and Hantzsch reactions using nanoZnO as a structure base catalyst. J Mol Catal A Chem 370:117–122. https://doi.org/10.1016/j.molcata.2012.12.005
Amini MM, Shaabani A, Bazgir A (2006) Tangstophosphoric acid (\(\text{ H }_{3}\text{ PW }_{12}\text{ O }_{40})\): an efficient and eco-friendly catalyst for the one-pot synthesis of dihydropyrimidin-2(1H)-ones. Catal Commun 7:843–847. https://doi.org/10.1016/j.catcom.2006.02.027
Singh OM, Singh SJ, Devi MB, Devi LN, Singh NI, Lee SG (2008) Synthesis and in vitro evaluation of the antifungal activities of dihydropyrimidinones. Bioorg Med Chem Lett 18:6462–6467. https://doi.org/10.1016/j.bmcl.2008.10.063
Verma S, Jain SL, Sain B (2010) PEG-embedded thiourea dioxide (PEG.TUD) as a novel organocatalyst for the highly efficient synthesis of 3,4-dihydropyrimidinones. Tetrahedron Lett 51:6897–6900. https://doi.org/10.1016/j.tetlet.2010.10.124
Reddy BM, Sreekanth PM, Lakshmanan P (2005) Sulfated zirconia as an efficient catalyst for organic synthesis and transformation reactions. J Mol Catal A: Chem 237:93–100. https://doi.org/10.1016/j.molcata.2005.04.039
Qiu Y, Sun H, Ma Z, Xia W (2014) Efficient, stable, and reusable Lewis acid-surfactant-combined catalyst: one-pot Biginelli and solvent-free esterification reactions. J Mol Catal A Chem 392:76–82. https://doi.org/10.1016/j.molcata.2014.04.031
Bose AK, Pednekar S, Ganguly SN, Chakraborty G, Manhas MS (2004) A simplified green chemistry approach to the Biginelli reaction using ‘Grindstone Chemistry’. Tetrahedron Lett 45:8351–8353. https://doi.org/10.1016/j.tetlet.2004.09.064
Prodius D, Macaev F, Mereacre V, Shova S, Lutsenco Y, Styngach E, Ruiz P, Muraviev D, Lipkowski J, Simonov YA, Turta C (2009) Synthesis and characterization of \(\{\text{ Fe }_{2}\text{ CuO }\}\) clusters as precursors for nanosized catalytic system for Biginelli reaction. Inorg Chem Commun 12:642–645. https://doi.org/10.1016/j.inoche.2009.05.011
Moghaddas M, Davoodnia A, Heravi MM, Tavakoli-Hoseini N (2012) Sulfonated carbon catalyzed Biginelli reaction for one-pot synthesis of 3,4-dihydropyrimidin-2 (1H)-ones and-thiones. Chin J Catal 33:706–710. https://doi.org/10.1016/S1872-2067(11)60377-X
Jetti SR, Bhatewara A, Kadre T, Jain S (2014) Silica-bonded N-propyl sulfamic acid as an efficient recyclable catalyst for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under heterogeneous conditions. Chin Chem Lett 25:469–473. https://doi.org/10.1016/j.cclet.2013.12.022
Rostamnia S, Lamei K (2012) Diketene-based neat four-component synthesis of the dihydropyrimidinones and dihydropyridine backbones using silica sulfuric acid (SSA). Chin Chem Lett 23:930–932. https://doi.org/10.1016/j.cclet.2012.06.008
Ábrányi-Balogh P, Dancsó A, Frigyes D, Volk B, Keglevich G, Milen M (2014) Convenient synthesis of 1-aryl-9H-\(\upbeta \)-carboline-3-carbaldehydes andtheir transformation into dihydropyrimidinone derivatives by Biginelli reaction. Tetrahedron 70:5711–5719. https://doi.org/10.1016/j.tet.2014.06.073
Fazaeli R, Tangestaninejad S, Aliyan H, Moghadam M (2006) One-pot synthesis of dihydropyrimidinones using facile and reusable polyoxometalate catalysts for the Biginelli reaction. Appl Catal A Gen 309:44–51. https://doi.org/10.1016/j.apcata.2006.04.043
Saher L, Makhloufi-Chebli M, Dermeche L, Boutemeur-Khedis B, Rabia C, Silva AMS, Hamdi M (2016) Keggin and Dawson-type polyoxometalates as efficient catalysts for the synthesis of 3,4-dihydropyrimidinones: experimental and theoretical studies. Tetrahedron Lett 57:1492–1496. https://doi.org/10.1016/j.tetlet.2016.02.077
Benazzouz A, Makhloufi-Chebli M, Khatir-Hamdi N, Boutemeur-Khedis B, Silva AM, Hamdi M (2015) A facile synthesis of new coumarin-3,4-dihydropyrimidin-2(1H)-ones/thiones dyads. Tetrahedron 71:3890–3894. https://doi.org/10.1016/j.tet.2015.04.028
Rao GBD, Anjaneyulu B, Kaushik MP (2014) A facile one-pot five-component synthesis of glycoside annulated dihydropyrimidinone derivatives with 1,2,3-triazol linkage via transesterification/Biginelli/click reactions in aqueous medium. Tetrahedron Lett 55:19–22. https://doi.org/10.1016/j.tetlet.2013.09.023
Arunkhamkaew S, Athipornchai A, Apiratikul N, Suksamrarn A, Ajavakom V (2013) Novel racemic tetrahydrocurcuminoid dihydropyrimidinone analogues as potent acetylcholinesterase inhibitors. Bioorg Med Chem Lett 23:2880–2882. https://doi.org/10.1016/j.bmcl.2013.03.069
Fu NY, Yuan YF, Pang ML, Wang JT, Peppe C (2003) Indium(III) halides-catalyzed preparation of ferrocene-dihydropyrimidinones. J Org Chem 672:52–57. https://doi.org/10.1016/S0022-328X(03)00139-6
Rafiee E, Jafari H (2006) A practical and green approach towards synthesis of dihydropyrimidinones: using heteropoly acids as efficient catalysts. Bioorg Med Chem Lett 16:2463–2466. https://doi.org/10.1016/j.bmcl.2006.01.087
Fustero S, Catalán S, Aceña JL, Pozo CD (2009) A new strategy for the synthesis of fluorinated 3,4-dihydropyrimidinones. J Fluorine Chem 130:1145–1150. https://doi.org/10.1016/j.jfluchem.2009.06.001
Fu NY, Yuan YF, Cao Z, Wang SW, Wang JT, Peppe C (2002) Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: improved protocol conditions for the Biginelli reaction. Tetrahedron 58:4801–4807. https://doi.org/10.1016/S0040-4020(02)00455-6
Cepanec I, Litvić M, Bartolinčić A, Lovrić M (2005) Ferric chloride/tetraethyl orthosilicate as an efficient system for synthesis of dihydropyrimidinones by Biginelli reaction. Tetrahedron 61:4275–4280. https://doi.org/10.1016/j.tet.2005.02.059
Cepanec I, Litvić M, Filipan-Litvić M, Grüngold I (2007) Antimony(III) chloride-catalysed Biginelli reaction: a versatile method for the synthesis of dihydropyrimidinones through a different reaction mechanism. Tetrahedron 63:11822–11827. https://doi.org/10.1016/j.tet.2007.09.045
Singh K, Singh S (2008) Antimony(III) chloride-catalysed Biginelli reaction: a versatile method for the synthesis of dihydropyrimidinones through a different reaction mechanism. Tetrahedron 64:11718–11723. https://doi.org/10.1016/j.tet.2007.09.045
Lu J, Bai Y, Wang Z, Yang B, Ma H (2000) One-pot synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones using lanthanum chloride as a catalyst. Tetrahedron Lett 41:9075–9078. https://doi.org/10.1016/S0040-4039(00)01645-2
Reddy CV, Mahesh M, Raju PVK, Babu TR, Reddy VVN (2002) Zirconium(IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones. Tetrahedron Lett 43:2657–2659. https://doi.org/10.1016/S0040-4039(02)00280-0
Maiti G, Kundu P, Guin C (2003) One-pot synthesis of dihydropyrimidinones catalysed by lithium bromide: an improved procedure for the Biginelli reaction. Tetrahedron Lett 44:2757–2758. https://doi.org/10.1016/S0040-4039(02)02859-9
Salehi P, Dabiri M, Zolfigol MA, Bodaghi Fard MA (2003) Silica sulfuric acid: an efficient and reusable catalyst for the one-pot synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones. Tetrahedron Lett 44:2889–2891. https://doi.org/10.1016/S0040-4039(03)00436-2
Abelman MM, Smith SC, James DR (2003) Cyclic ketones and substituted \(\upalpha \)-keto acids as alternative substrates for novel Biginelli-like scaffold syntheses. Tetrahedron Lett 44:4559–4562. https://doi.org/10.1016/S0040-4039(03)00985-7
Bhosale RS, Bhosale SV, Bhosale SV, Wang T, Zubaidha PK (2004) An efficient, high yield protocol for the one-pot synthesis of dihydropyrimidin-2\((1H)\)-ones catalyzed by iodine. Tetrahedron Lett 45:9111–9113. https://doi.org/10.1016/j.tetlet.2004.10.021
Bose AK, Manhas MS, Pednekar S, Ganguly SN, Dang H, He W, Mandadi A (2005) Large scale Biginelli reaction via water-based biphasic media: a green chemistry strategy. Tetrahedron Lett 46:1901–1903. https://doi.org/10.1016/j.tetlet.2005.01.087
Mabry J, Ganem B (2006) Studies on the Biginelli reaction: a mild and selective route to 3,4-dihydropyrimidin-2\((1H)\)-ones via enamine intermediates. Tetrahedron Lett 47:55–56. https://doi.org/10.1016/j.tetlet.2005.10.124
Mandhane PG, Joshi RS, Nagargoje DR, Gill CH (2010) An efficient synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones catalyzed by thiamine hydrochloride in water under ultrasound irradiation. Tetrahedron Lett 51:3138–3140. https://doi.org/10.1016/j.tetlet.2010.04.037
Russowsky D, Canto RFS, Sanches SAA, Doca MGM, Fátima AD, Pilli RA, Kohn LK, Antônio MA, Carvalho JED (2006) Synthesis and differential antiproliferative activity of Biginelli compounds against cancer cell lines: monastrol, oxo-monastrol and oxygenated analogues. Bioorg Chem 34:173–182. https://doi.org/10.1016/j.bioorg.2006.04.003
Chitra S, Devanathan D, Pandiarajan K (2010) Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur J Med Chem 45:367–371. https://doi.org/10.1016/j.ejmech.2009.09.018
Lin HX, Zhao QJ, Xu B, Wang XH (2007) A green synthesis of dihydropyrimidinones by Biginelli reaction over Nafion-H catalyst. Chin Chem Lett 18:502–504. https://doi.org/10.1016/j.cclet.2007.03.022
Ghosh R, Maiti S, Chakraborty A (2004) In\((\text{ OTf })_{3}\)-catalysed one-pot synthesis of 3,4-dihydropyrimidin-2(lH)-ones. J Mol Catal A: Chem 217:47–50. https://doi.org/10.1016/j.molcata.2004.02.025
Zhang X, Li Y, Liu C, Wang J (2006) An efficient synthesis of 4-substituted pyrazolyl-3,4-dihydropyrimidin-2(1H)-(thio)ones catalyzed by \(\text{ Mg }(\text{ ClO }_{4})_{2}\) under ultrasound irradiation. J Mol Catal A: Chem 253:207–211. https://doi.org/10.1016/j.molcata.2006.03.018
Jain SL, Joseph JK, Singhal S, Sain B (2007) Metallophthalocyanines (MPcs) as efficient heterogeneous catalysts for Biginelli condensation: application and comparison in catalytic activity of different MPcs for one pot synthesis of 3,4-dihydropyrimidin-2-\((1H)\)-ones. J Mol Catal A Chem 268:134–138. https://doi.org/10.1016/j.molcata.2006.12.015
Rajack A, Yuvaraju K, Praveen C, Murthy YLN (2013) A facile synthesis of 3,4-dihydropyrimidinones/thiones and novel \(N\)-dihydro pyrimidinone-decahydroacridine-1,8-diones catalyzed by cellulose sulfuric acid. J Mol Catal A Chem 370:197–204. https://doi.org/10.1016/j.molcata.2013.01.003
Heravi MM, Bakhtiari K, Bamoharram FF (2006) 12-Molybdophosphoric acid: a recyclable catalyst for the synthesis of Biginelli-type 3,4-dihydropyrimidine-2(1H)-ones. Catal Commun 7:373–376. https://doi.org/10.1016/j.catcom.2005.12.007
Barrow JC, Glass KL, Selnick HG, Freidinger RM, Chang RSL, O’Malley SS, Woyden C (2000) Preparation and evaluation of 1,3-diaminocyclopentane-linked dihydropyrimidinone derivatives as selective \(\upalpha \)1a-receptor antagonists. Bioorg Med Chem Lett 10:1917–1920. https://doi.org/10.1016/S0960-894X(00)00374-7
Sabitha G, Reddy GSKK, Reddy KB, Yadav JS (2003) Vanadium(III) chloride catalyzed Biginelli condensation: solution phase library generation of dihydropyrimidin-\((2H)\)-ones. Tetrahedron Lett 44:6497–6499. https://doi.org/10.1016/S0040-4039(03)01564-8
Jenner G (2004) Effect of high pressure on Biginelli reactions. Steric hindrance and mechanistic considerations. Tetrahedron Lett 45:6195–6198. https://doi.org/10.1016/j.tetlet.2004.05.106
Frija LMT, Khmelinskii IV, Cristiano MLS (2005) Novel efficient synthesis of 3,4-dihydro-6-substituted-3-phenylpyrimidin-2\((1H)\)-ones. Tetrahedron Lett 46:6757–6760. https://doi.org/10.1016/j.tetlet.2005.07.101
Sabitha G, Reddy KB, Yadav JS, Shailaja D, Sivudu KS (2005) Ceria/vinylpyridine polymer nanocomposite: an ecofriendly catalyst for the synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones. Tetrahedron Lett 46:8221–8224. https://doi.org/10.1016/j.tetlet.2005.09.100
Debache A, Boumoud B, Amimour M, Belfaitah A, Rhouati S, Carboni B (2006) Phenylboronic acid as a mild and efficient catalyst for Biginelli reaction. Tetrahedron Lett 47:5697–5699. https://doi.org/10.1016/j.tetlet.2006.06.015
Suzuki I, Suzumura Y, Takeda K (2006) Metal triflimide as a Lewis acid catalyst for Biginelli reactions in water. Tetrahedron Lett 47:7861–7864. https://doi.org/10.1016/j.tetlet.2006.09.019
Singh K, Arora D, Singh S (2007) A highly regio- and chemoselective addition of carbon nucleophiles to pyrimidinones. A new route to C4 elaborated Biginelli compounds. Tetrahedron Lett 48:1349–1352. https://doi.org/10.1016/j.tetlet.2006.12.111
Tamaddon F, Razmi Z, Jafari AA (2010) Synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones and 1,4-dihydropyridines using ammonium carbonate in water. Tetrahedron Lett 51:1187–1189. https://doi.org/10.1016/j.tetlet.2009.12.098
Konkala K, Sabbavarapu NM, Katla R, Durga NYV, Reddy TVK, Devi B, Rachapudi BNP (2012) Revisit to the Biginelli reaction: a novel and recyclable bioglycerol-based sulfonic acid functionalized carbon catalyst for one-pot synthesis of substituted 3,4-dihydropyrimidin-2-\((1H)\)-ones. Tetrahedron Lett 53:1968–1973. https://doi.org/10.1016/j.tetlet.2012.02.018
Singh K, Singh K, Trappanese DM, Moreland RS (2012) Highly regioselective synthesis of N-3 organophosphorous derivatives of3,4-dihydropyrimidin-2\((1H)\)-ones and their calcium channel binding studies. Eur J Med Chem 54:397–402. https://doi.org/10.1016/j.ejmech.2012.05.017
Treptow TGM, Figueiró F, Jandrey EHF, Battastini AMO, Salbego CG, Hoppe JB, Taborda PS, Rosa SB, Piovesan LA (2015) Novel hybrid DHPM-fatty acids: synthesis and activity against glioma cell growth invitro. Eur J Med Chem 95:552–562. https://doi.org/10.1016/j.ejmech.2015.03.062
Ghorbani-Choghamarani A, Zamani P (2013) Three component reactions: an efficient and green synthesis of 3,4-dihydropyrimidin-2-\((1H)\)-ones and thiones using silica gel-supported l-pyrrolidine-2-carboxylic acid-4-hydrogen sulfate. Chin Chem Lett 24:804–808. https://doi.org/10.1016/j.cclet.2013.05.033
Gupta P, Paul S (2012) Sulfonated carbon/silica composite functionalized Lewis acids for one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles, 3,4-dihydropyrimidin-2\((1H)\)-ones and for Michael addition of indole to \(\upalpha \),\(\upbeta \)-unsaturated ketones. J Mol Catal A: Chem 352:75–80. https://doi.org/10.1016/j.molcata.2011.10.016
Ahmed N, Siddiqui ZN (2014) Sulphated silica tungstic acid as a highly efficient and recyclable solid acid catalyst for the synthesis of tetrahydropyrimidines and dihydropyrimidines. J Mol Catal A: Chem 387:45–56. https://doi.org/10.1016/j.molcata.2014.02.019
Medyouni R, Elgabsi W, Naouali O, Romerosa A, Al-Ayed AS, Baklouti L, Hamdi N (2016) One-pot three-component Biginelli-type reaction to synthesize 3,4-dihydropyrimidine-2-(1H)-ones catalyzed by Co phthalocyanines: Synthesis, characterization, aggregation behavior and antibacterial activity. Spectrochim Acta A167:165–174. https://doi.org/10.1016/j.saa.2016.04.045
Maradur SP, Gokavi GS (2007) Heteropoly acid catalyzed synthesis of 3,4-dihydropyrimidin-2\((1H)\)-ones. Catal Commun 8:279–284. https://doi.org/10.1016/j.catcom.2006.05.048
Savanur HM, Kalkhambkar RG, Aridoss G, Laali KK (2016) [bmim(\(\text{ SO }_{3}\)H)][OTf]/[bmim][X] and Zn(\(\text{ NTf }_{2})_{2}\)/[bmim][X] (X = \(\text{ PF }_{6}\) and \(\text{ BF }_{4})\); efficient catalytic systems for the synthesis of tetrahydropyrimidin-ones (-thiones) via the Biginelli reaction. Tetrahedron Lett 57:3029–3035. https://doi.org/10.1016/j.tetlet.2016.05.103
Titova Y, Fedorova O, Rusinov G, Vigorov A, Krasnov V, Murashkevich A, Charushin V (2015) Effect of nanosized \(\text{ TiO }_{2}\)-\(\text{ SiO }_{2}\) covalently modified by chiral molecules on the asymmetric Biginelli reaction. Catal Today 241:270–274. https://doi.org/10.1016/j.cattod.2014.01.035
Steele TG, Coburn CA, Patane MA, Bock MG (1998) Expedient synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2\((1H)\)-ones. Tetrahedron Lett 39:9315–9318. https://doi.org/10.1016/S0040-4039(98)02155-8
Kumar A, Maurya RA (2007) An efficient bakers’ yeast catalyzed synthesis of 3,4-dihydropyrimidin-2-\((1H)\)-ones. Tetrahedron Lett 48:4569–4571. https://doi.org/10.1016/j.tetlet.2007.04.130
Joseph JK, Jain SL, Sain B (2006) Ion exchange resins as recyclable and heterogeneous solid acid catalysts for the Biginelli condensation: an improved protocol for the synthesis of 3,4-dihydropyrimidin-2-ones. J Mol Catal A Chem 247:99–102. https://doi.org/10.1016/j.molcata.2005.11.028
Shen P, Xu M, Yin D, Xie S, Zhou C, Li F (2016) Halogenated macroporous sulfonic resins as efficient catalysts for the Biginelli reaction. Catal Commun 77:18–21. https://doi.org/10.1016/j.catcom.2016.01.010
Saloutin I, Burgart YV, Kuzueva OG, Kappe CO, Chupakhin ON (2000) Biginelli condensations of fluorinated 3-oxo esters and 1,3-diketones. J Fluorine Chem 103:17–23. https://doi.org/10.1016/S0022-1139(99)00216-X
Ryabukhin SV, Plaskon AS, Ostapchuk EN, Volochnyuk DM, Shishkin OV, Tolmachev AA (2008) \(\text{ CF }_{3}\)-substituted 1,3-dicarbonyl compounds in the Biginelli reaction promoted by chlorotrimethylsilane. J Fluorine Chem 129:625–631. https://doi.org/10.1016/j.jfluchem.2008.05.004
Legeay JC, Vanden E, Bazureau JP (2008) Ionic liquid phase organic synthesis (IoLiPOS) methodology applied to the preparation of new 3,4-dihydropyrimidine-2\((1H)\)-ones bearing bioisostere group in N-3 position. Tetrahedron 64:5328–5335. https://doi.org/10.1016/j.tet.2008.03.021
Legeay JC, Vanden Eynde JJ, Bazureau JP (2007) A new approach to N-3 functionalized 3,4-dihydropyrimidine-2\((1H)\)-ones with 1,2,4-oxadiazole group as amide isostere via ionic liquid-phase technology. Tetrahedron Lett 48:1063–1068. https://doi.org/10.1016/j.tetlet.2006.11.148
Shaabani A, Seyyedhamzeh M, Maleki A, Hajishaabanha F (2010) Diketene as an alternative substrate for a new Biginelli-like multicomponent reaction: one-pot synthesis of 5-carboxamide substituted 3,4-dihydropyrimidine-2\((1H)\)ones. Tetrahedron 66:4040–4042. https://doi.org/10.1016/j.tet.2010.04.028
Xu DZ, Li H, Wang Y (2012) Highly enantioselective Biginelli reaction catalyzed by a simple chiral primary amine catalyst: asymmetric synthesis of dihydropyrimidines. Tetrahedron 68:7867–7872. https://doi.org/10.1016/j.tet.2012.07.027
Zhang A, Zhang L, Duan X, Yan X, Yan Y, Liu Q, Liu T, Zhang G (2015) Iron-catalyzed four-member multicomponent reaction for assembly of (\(E)\)-6-arylvinyl-3,4-dihydropyrimidin-2\((1H)\)-ones. Tetrahedron 71:7745–7751. https://doi.org/10.1016/j.tet.2015.07.039
Suzuki I, Iwata Y, Takeda K (2008) Biginelli reactions catalyzed by hydrazine type organocatalyst. Tetrahedron Lett 49:3238–3241. https://doi.org/10.1016/j.tetlet.2008.03.080
Zych AJ, Wang HJ, Sakwa SA (2010) Synthesis and Suzuki–Miyaura reactions of 5-halo-3,4-dihydropyrimidin-2\((1H)\)-ones. Tetrahedron Lett 51:5103–5105. https://doi.org/10.1016/j.tetlet.2010.07.093
Rao GBD, Acharya BN, Verma SK, Kaushik MP (2011) \(N\),\(N^\prime \)-Dichlorobis(2,4,6-trichlorophenyl)urea (CC-2) as a new reagent for the synthesis of pyrimidone and pyrimidine derivatives via Biginelli reaction. Tetrahedron Lett 52:809–812. https://doi.org/10.1016/j.tetlet.2010.12.039
Kolosov MA, Kulyk OG, Al-Ogaili MJK, Orlov VD (2015) An effective Biginelli-type synthesis of 1-methoxy-3,4-dihydropyrimidin-2\((1H)\)-ones. Tetrahedron Lett 56:4666–4669. https://doi.org/10.1016/j.tetlet.2015.06.041
Zorkun S, Saraç S, Çelebi S, Erol K (2006) Synthesis of 4-aryl-3,4-dihydropyrimidin-2\((1H)\)-thione derivatives as potential calcium channel blockers. Bioorg Med Chem 14:8582–8589. https://doi.org/10.1016/j.bmc.2006.08.031
Siddiqui ZN (2013) Bis[(L)prolinato-N, O]Zn-water: a green catalytic system for the synthesis of 3,4-dihydropyrimidin-2 (\(1H)\)-ones via the Biginelli reaction. CR Chim 16:183–188. https://doi.org/10.1016/j.crci.2012.10.008
Wang X, Quan Z, Wang JK, Zhang Z, Wang M (2006) A practical and green approach toward synthesis of \(N3\)-substituted dihydropyrimidinones: using Aza-Michael addition reaction catalyzed by \(\text{ KF }/\text{ Al }_{2}\text{ O }_{3}\). Bioorg Med Chem Lett 16:4592–4595. https://doi.org/10.1016/j.bmcl.2006.06.006
Hamper BC, Owen TJ (1999) Solid phase synthesis of dihydropyrimidinones and pyrimidinone carboxylic acids from malonic acid resin. Tetrahedron Lett 40:4973–4976. https://doi.org/10.1016/S0040-4039(99),00961-2
Lacotte P, Buisson DA, Ambroise Y (2013) Synthesis, evaluation and absolute configuration assignment of novel dihydropyrimidin-2-ones as picomolar sodium iodide symporter inhibitors. Eur J Med Chem 62:722–727. https://doi.org/10.1016/j.ejmech.2013.01.043
Ryabukhin SV, Plaskon AS, Bondarenko SS, Ostapchuk EN, Grygorenko OO, Shishkin OV, Tolmachev AA (2010) Acyl pyruvates as synthons in the Biginelli reaction. Tetrahedron Lett 51:4229–4232. https://doi.org/10.1016/j.tetlet.2010.06.032
Singh K, Singh S (2006) A mild and practical method for the regioselective synthesis of N-acylated 3,4-dihydropyrimidin-2-ones. New acyl transfer reagents. Tetrahedron Lett 47:8143–8146. https://doi.org/10.1016/j.tetlet.2006.09.039
Kappe CO (2000) Highly versatile solid phase synthesis of biofunctional 4-aryl-3,4-dihydropyrimidines using resin-bound isothiourea building blocks and multidirectional resin cleavage. Bioorg Med Chem Lett 10:49–51. https://doi.org/10.1016/S0960-894X(99)00572-7
Kiss K, Csámpai A, Sohár P (2010) New ferrocenyl-substituted heterocycles. Formation under Biginelli conditions, DFT modelling, and structure determination. J Organomet Chem 695:1852–1857. https://doi.org/10.1016/j.jorganchem.2010.04.036
Savithri A, Chinnan CN, Varma L (2015) Synthesis of dihydropyrimidine derivatives of calix[4]arene via adaptation of Biginelli-3-component reaction. Tetrahedron 71:9667–9672. https://doi.org/10.1016/j.tet.2015.10.066
Wang G, Yan C, Wang D, Li D, Lu Y (2012) Specific binding of a dihydropyrimidinone derivative with DNA: spectroscopic, calorimetric and modeling investigations. J Lumin 132:1656–1662. https://doi.org/10.1016/j.jlumin.2012.02.021
Stefani HA, Oliveira CB, Almeida RB, Pereira CMP, Braga RC, Cella R, Borges VC, Savegnago L, Nogueira CW (2006) Dihydropyrimidin-(\(2H\))-ones obtained byultrasound irradiation: anew class ofpotential antioxidant agents. Eur J MedChem 41:513–518. https://doi.org/10.1016/j.ejmech.2006.01.007
Schmidt RJ, Lombardo LJ, Traeger SC, Williams DK (2008) One-pot two step synthesis of 5-cyano-dihydropyrimidinones using polyphosphate ester. Tetrahedron Lett 49:3009–3010. https://doi.org/10.1016/j.tetlet.2008.02.162
Pasunooti KK, Chai H, Jensen CN, Gorityala BK, Wang S, Liu XW (2011) A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions. Tetrahedron Lett 52:80–84. https://doi.org/10.1016/j.tetlet.2010.10.150
Li JT, Han JF, Yang JH, Li TS (2003) An efficient synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by \(\text{ NH }_{2}\text{ SO }_{3}\text{ H }\) under ultrasound irradiation. Ultrason Sonochem 10:119–122. https://doi.org/10.1016/S1350-4177(03)00092-0
Tajbakhsh M, Mohajerani B, Heravi MM, Ahmadi AN (2005) Natural HEU type zeolite catalyzed Biginelli reaction for the synthesis of 3,4-dihydropyrimidin-2(1H) one derivatives. J Mol Catal A Chem 236:216–219. https://doi.org/10.1016/j.molcata.2005.04.033
Peng J, Deng Y (2001) Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Lett 42:5917–5919. https://doi.org/10.1016/S0040-4039(01)01139-X
Swatlowski RP, Rogers RD, Holbrey RD (2003) Ionic liquids are not always green: hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem 5:361–363. https://doi.org/10.1039/B304400A
Li M, Guo WS, Wen LR, Li YF, Yang HZ (2006) One-pot synthesis of Biginelli and Hantzsch products catalyzed by non-toxic ionic liquid (BMImSac) and structural determination of two products. J Mol Catal A Chem 258:133–138. https://doi.org/10.1016/j.molcata.2006.05.028
Quan ZJ, Da Y-X, Zhang Z, Wang XC (2009) PS-PEG-\(\text{ SO }_{3}\text{ H }\) as an efficient catalyst for 3,4-dihydropyrimidones via Biginelli reaction. Catal Commun 10:1146–1148. https://doi.org/10.1016/j.catcom.2008.12.017
Bahekar SS, Kotharkar SA, Shinde DB (2004) One-pot construction of dihydropyrimidinones in ionic liquids. Mendeleev Commun 14:210–212. https://doi.org/10.1070/MC2004v014n05ABEH001895
Dondoni A, Massi A, Sabbatini S (2001) Towards the synthesis of C-glycosylated dihydropyrimidine libraries via the three-component Biginelli reaction. A novel approach to artificial nucleosides. Tetrahedron Lett 42(2001):4495–4497. https://doi.org/10.1016/S0040-4039(01)00769-9
Zumpe FL, Flüß M, Schmitz K, Lender A (2007) Propane phosphonic acid anhydride: a new promoter for the one-pot Biginelli synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett 48:1421–1423. https://doi.org/10.1016/j.tetlet.2006.12.098
Garima VPS, Yadav LDS (2010) Biginelli reaction starting directly from alcohols. Tetrahedron Lett 51:6436–6438. https://doi.org/10.1016/j.tetlet.2010.09.141
Timoshenko VM, Markitanov YM, Shermolovich YG (2011) 2-Oxo-2-polyfluoroalkylethane-1-sulfones and -sulfamides in the Biginelli and ‘retro-Biginelli’ reactions. Tetrahedron Lett 52:6619–6622. https://doi.org/10.1016/j.tetlet.2011.09.143
Singh K, Arora D, Poremsky E, Lowery J, Moreland RS (2009) N1-Alkylated 3,4-dihydropyrimidine-2(1H)-ones: convenient one-pot selective synthesis and evaluation of their calcium channel blocking activity. Eur J Med Chem 44:1997–2001. https://doi.org/10.1016/j.ejmech.2008.10.002
Zhang L, Xu L, Kim CU (2003) A new approach to the 2,5-diamino-5,6-dihydro-1H-pyrimidine-4-one derivatives: synthesis of TAN-1057A/B and analogs. Tetrahedron Lett 44:5871–5873. https://doi.org/10.1016/S0040-4039(03)01381-9
Ahmed B, Khan RA, Habibullah Keshari M (2009) An improved synthesis of Biginelli-type compounds via phase-transfer catalysis. Tetrahedron Lett 50:2889–2892. https://doi.org/10.1016/j.tetlet.2009.03.177
Tu S, Fang F, Miao C, Jiang H, Feng Y, Shi D, Wang X (2003) One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using boric acid as catalyst. Tetrahedron Lett 44:6153–6155. https://doi.org/10.1016/S0040-4039(03)01466-7
Wang ZT, Xu LW, Xia CG, Wang HQ (2004) Novel Biginelli-like three-component cyclocondensation reaction: efficient synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett 45:7951–7953. https://doi.org/10.1016/j.tetlet.2004.08.107
Chitra S, Pandiarajan K (2009) Calcium fluoride: an efficient and reusable catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and their corresponding 2(1H)thione: an improved high yielding protocol for the Biginelli reaction. Tetrahedron Lett 50:2222–2224. https://doi.org/10.1016/j.tetlet.2009.02.162
Starcevich JT, Laughlin TJ, Mohan RS (2013) Iron(III) tosylate catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via the Biginelli reaction. Tetrahedron Lett 54:983–985. https://doi.org/10.1016/j.tetlet.2012.12.032
Nagawade RR, Kotharkar SA, Shinde DB (2005) Titanium(IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin- 2(1H)-ones and thiones. Mendeleev Commun 15:150–151. https://doi.org/10.1070/MC2005v015n04ABEH002123.
Fang Z, Lam Y (2011) A rapid and convenient synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2-ones and thiones. Tetrahedron 67:1294–1297. https://doi.org/10.1016/j.tet.2010.11.075
Rodríguez-Domínguez JC, Bernardi D, Kirsch G (2007) \(\text{ ZrCl }_{4}\) or \(\text{ ZrOCl }_{2}\) under neat conditions: optimized green alternatives for the Biginelli reaction. Tetrahedron Lett 48:5777–5780. https://doi.org/10.1016/j.tetlet.2007.06.104
Paraskar AS, Dewkar GK, Sudalai A (2003) \(\text{ Cu }(\text{ OTf })_{2}\): a reusable catalyst for high-yield synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett 44:3305–3308. https://doi.org/10.1016/S0040-4039(03)00619-1
Yarapathi RV, Kurva S, Tammishetti S (2004) Synthesis of 3,4-dihydropyrimidin-2(1H)ones using reusable poly(4-vinylpyridine-co-divinylbenzene)-Cu(II)complex. Catal Commun 5:511–513. https://doi.org/10.1016/j.catcom.2004.06.007
Heravi MM, Derikvand F, Bamoharram FF (2005) A catalytic method for synthesis of Biginelli-type 3,4-dihydropyrimidin-2(1H)-one using 12-tungstophosphoric acid. J Mol Catal A Chem 242:173–175. https://doi.org/10.1016/j.molcata.2005.08.009
Kamal A, Malik MS, Bajee S, Azeeza S, Faazil S, Ramakrishna S, Naidu VGM, Vishnuwardhan MVPS (2011) Synthesis and biological evaluation of conformationally flexible as well as restricted dimers of monastrol and related dihydropyrimidones. Eur J Med Chem 46:3274–3281. https://doi.org/10.1016/j.ejmech.2011.04.048
Gijsen HJ, Berthelot D, De Cleyn MA, Geuens I, Brône B, Mercken M (2012) Tricyclic 3,4-dihydropyrimidine-2-thione derivatives as potent TRPA1 antagonists. Bioorg Med Chem Lett 22:797–800. https://doi.org/10.1016/j.bmcl.2011.12.068
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadi, B., Behbahani, F.K. Recent developments in the synthesis and applications of dihydropyrimidin-2(1H)-ones and thiones. Mol Divers 22, 405–446 (2018). https://doi.org/10.1007/s11030-017-9806-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-017-9806-z