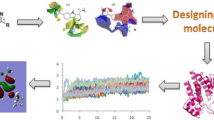
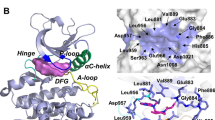
Abstract
Despite increase in the understanding of the pathogenesis of rheumatoid arthritis (RA), it remains a tough challenge. The advent of kinases involved in key intracellular pathways in pathogenesis of RA may provide a new phase of drug discovery for RA. The present study is aimed to identify dual JAK3/\(\hbox {PI3K}\delta \) inhibitors by developing an optimum pharmacophore model integrating the information revealed by ligand-based pharmacophore models and structure-based pharmacophore models (SBPMs). For JAK3 inhibitors, the addition of an aromatic ring feature and for \(\hbox {PI3K}\delta \) the addition of a hydrophobic feature proposed by SBPMs lead to five-point pharmacophore (i.e., AADHR.54 (JAK3)) and six-point pharmacophore (i.e., AAAHRR.45 (\(\hbox {PI3K}\delta \))). The obtained pharmacophores were validated and used for virtual screening and then for docking-based screening. Molecules were further evaluated for ADME properties, and their docked protein complexes were subjected to MM–GBSA energy calculations and molecular dynamic simulations. The top two hit compounds with novel scaffolds 2-oxo-1,2-dihydroquinoline and benzo[d]oxazole showed inhibitory activity for JAK3 and \(\hbox {PI3K}\delta \).











Similar content being viewed by others
References
Meier FM, McInnes IB (2014) Small-molecule therapeutics in rheumatoid arthritis: scientific rationale, efficacy and safety. Best Pract Res Clin Rheumatol 28:605–624. https://doi.org/10.1016/j.berh.2014.10.017
Chakravarty SD, Poulikakos PI, Ivashkiv LB, Salmon JE, Kalliolias GD (2013) Kinase inhibitors: a new tool for the treatment of rheumatoid arthritis. Clin Immunol 148:66–78. https://doi.org/10.1016/j.clim.2013.04.007
Fleur B, Elisa G, Peters GJ (2011) Tyrosine kinase inhibitors: multi-targeted or single-targeted? World J Clin Oncol 2:80–93. https://doi.org/10.5306/wjco.v2.i2.80
Papaetis GS, Syrigos KN (2009) Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. Bio Drugs 23:377–389. https://doi.org/10.2165/11318860-000000000-00000
McInnes IB, Schett G (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389:2328–2337. https://doi.org/10.1016/S0140-6736(17)31472-1
Cooles FA, Isaacs JD (2011) Pathophysiology of rheumatoid arthritis. Curr Opin Rheumatol 23:233–240. https://doi.org/10.1097/BOR.0b013e32834518a3
Norman P (2016) Investigational Bruton’s tyrosine kinase inhibitors for the treatment of rheumatoid arthritis. Expert Opin Inv Drug 25:891–899. https://doi.org/10.1080/13543784.2016.1182499
Brasca MG, Nesi M, Avanzi N, Ballinari D, Bandiera T, Bertrand J, Bindi S, Canevari G, Carenzi D, Casero D, Ceriani L (2014) Pyrrole-3-carboxamides as potent and selective JAK2 inhibitors. Bioorg Med Chem 17:4998–5012. https://doi.org/10.1097/BOR.0b013e32834518a3
Brown GR, Bamford AM, Bowyer J, James DS, Rankine N, Tang E, Torr V, Culbert EJ (2000) Naphthyl ketones: a new class of Janus kinase 3 inhibitors. Bioorg Med Chem Lett 6:575–579. https://doi.org/10.1016/S0960-894X(00)00051-2
Duan JJ, Lu Z, Jiang B, Yang BV, Doweyko LM, Nirschl DS, Haque LE, Lin S, Brown G, Hynes J, Tokarski JS (2014) Discovery of pyrrolo[1,2-b]pyridazine-3-carboxamides as Janus kinase (JAK) inhibitors. Bioorg Med Chem Lett 24:5721–5726. https://doi.org/10.1016/j.bmcl.2014.10.061
de Vicente J, Lemoine R, Bartlett M, Hermann JC, Hekmat-Nejad M, Henningsen R, Jin S, Kuglstatter A, Li H, Lovey AJ, Menke J (2014) Scaffold hopping towards potent and selective JAK3 inhibitors: discovery of novel C-5 substituted pyrrolopyrazines. Bioorg Med Chem Lett 24:4969–4975. https://doi.org/10.1016/j.bmcl.2014.09.031
McDonnell ME, Bian H, Wrobel J, Smith GR, Liang S, Ma H, Reitz AB (2014) Anilino-monoindolylmaleimides as potent and selective JAK3 inhibitors. Bioorg Med Chem Lett 24:1116–1121. https://doi.org/10.1016/j.bmcl.2014.01.001
Jaime-Figueroa S, De Vicente J, Hermann J, Jahangir A, Jin S, Kuglstatter A, Lynch SM, Menke J, Niu L, Patel V, Shao A (2013) Discovery of a series of novel 5H-pyrrolo[2,3-b]pyrazine-2-phenyl ethers, as potent JAK3 kinase inhibitors. Bioorg Med Chem Lett 23:2522–2526. https://doi.org/10.1016/j.bmcl.2013.03.015
Lynch SM, DeVicente J, Hermann JC, Jaime-Figueroa S, Jin S, Kuglstatter A, Li H, Lovey A, Menke J, Niu L, Patel V (2013) Strategic use of conformational bias and structure based design to identify potent JAK3 inhibitors with improved selectivity against the JAK family and the kinome. Bioorg Med Chem Lett 23:2793–2800. https://doi.org/10.1016/j.bmcl.2013.02.012
Forsyth T, Kearney PC, Kim BG, Johnson HW, Aay N, Arcalas A, Brown DS, Chan V, Chen J, Du H, Epshteyn S (2012) SAR and in vivo evaluation of 4-aryl-2-aminoalkylpyrimidines as potent and selective Janus kinase 2 (JAK2) inhibitors. Bioorg Med Chem Lett 22:7653–7658. https://doi.org/10.1016/j.bmcl.2012.10.007
Harikrishnan LS, Kamau MG, Wan H, Inghrim JA, Zimmermann K, Sang X, Mastalerz HA, Johnson WL, Zhang G, Lombardo LJ, Poss MA (2011) Pyrrolo[1,2-f]triazines as JAK2 inhibitors: achieving potency and selectivity for JAK2 over JAK3. Bioorg Med Chem Lett 21:1425–1428. https://doi.org/10.1016/j.bmcl.2011.01.022
Malerich JP, Lam JS, Hart B, Fine RM, Klebansky B, Tanga MJ, D’Andrea A (2010) Diamino-1,2,4-triazole derivatives are selective inhibitors of TYK2 and JAK1 over JAK2 and JAK3. Bioorg Med Chem Lett 20:7454–7457. https://doi.org/10.1016/j.bmcl.2010.10.026
Pissot-Soldermann C, Gerspacher M, Furet P, Gaul C, Holzer P, McCarthy C, Radimerski T, Regnier CH, Baffert F, Drueckes P, Tavares GA (2010) Discovery and SAR of potent, orally available 2,8-diaryl-quinoxalines as a new class of JAK2 inhibitors. Bioorg Med Chem Lett 20:2609–2613. https://doi.org/10.1016/j.bmcl.2010.02.056
Ioannidis S, Lamb ML, Wang T, Almeida L, Block MH, Davies AM, Peng B, Su M, Zhang HJ, Hoffmann E, Rivard C (2009) Discovery of pyrazol-3-ylamino pyrazines as novel JAK2 inhibitors. Bioorg Med Chem Lett 19:6524–6528. https://doi.org/10.1016/j.bmcl.2009.10.054
Cole AG, Bohnstedt AC, Paradkar V, Kingsbury C, Quintero JG, Park H, Lu Y, You M, Neagu I, Diller DJ, Letourneau JJ (2009) 2-Benzimidazolyl-9-(chroman-4-yl)-purinone derivatives as JAK3 inhibitors. Bioorg Med Chem Lett 19:6788–6792. https://doi.org/10.1016/j.bmcl.2009.09.080
Burns CJ, Bourke DG, Andrau L, Bu X, Charman SA, Donohue AC, Fantino E, Farrugia M, Feutrill JT, Joffe M, Kling MR (2009) Phenylaminopyrimidines as inhibitors of Janus kinases (JAKs). Bioorg Med Chem Lett 19:5887–5892. https://doi.org/10.1016/j.bmcl.2009.08.071
Clark MP, George KM, Bookland RG, Chen J, Laughlin SK, Thakur KD, Lee W, Davis JR, Cabrera EJ, Brugel TA, VanRens JC (2007) Development of new pyrrolopyrimidine-based inhibitors of Janus kinase 3 (JAK3). Bioorg Med Chem Lett 17:1250–1253. https://doi.org/10.1016/j.bmcl.2006.12.018
Chen JJ, Thakur KD, Clark MP, Laughlin SK, George KM, Bookland RG, Davis JR, Cabrera EJ, Easwaran V, De B, Zhang YG (2006) Development of pyrimidine-based inhibitors of Janus tyrosine kinase 3. Bioorg Med Chem Lett 16:5633–5638. https://doi.org/10.1016/j.bmcl.2006.08.022
Gerspacher M, Furet P, Pissot-Soldermann C, Gaul C, Holzer P, Vangrevelinghe E, Lang M, Erdmann D, Radimerski T, Regnier CH, Chene P (2010) 2-Amino-aryl-7-aryl-benzoxazoles as potent, selective and orally available JAK2 inhibitors. Bioorg Med Chem Lett 20:1724–1727. https://doi.org/10.1016/j.bmcl.2010.01.069
Yang SM, Malaviya R, Wilson LJ, Argentieri R, Chen X, Yang C, Wang B, Cavender D, Murray WV (2007) Simplified staurosporine analogs as potent JAK3 inhibitors. Bioorg Med Chem Lett 17:326–331. https://doi.org/10.1016/j.bmcl.2006.10.062
Thoma G, Drückes P, Zerwes HG (2014) Selective inhibitors of the Janus kinase Jak3—are they effective? Bioorg Med Chem Lett 24:4617–4621. https://doi.org/10.1016/j.bmcl.2014.08.046
Adams C, Aldous DJ, Amendola S, Bamborough P, Bright C, Crowe S, Eastwood P, Fenton G, Foster M, Harrison TK, King S (2003) Mapping the kinase domain of Janus Kinase 3. Bioorg Med Chem Lett 13:3105–3110. https://doi.org/10.1016/S0960-894X(03)00657-7
Alexander R, Balasundaram A, Batchelor M, Brookings D, Crépy K, Crabbe T, Deltent MF, Driessens F, Gill A, Harris S, Hutchinson G (2008) 4-(1,3-Thiazol-2-yl)morpholine derivatives as inhibitors of phosphoinositide 3-kinase. Bioorg Med Chem Lett 18:4316–4320. https://doi.org/10.1016/j.bmcl.2008.06.076
Frédérick R, Mawson C, Kendall JD, Chaussade C, Rewcastle GW, Shepherd PR, Denny WA (2009) Phosphoinositide-3-kinase (PI3K) inhibitors: identification of new scaffolds using virtual screening. Bioorg Med Chem Lett 19:5842–5847. https://doi.org/10.1016/j.bmcl.2009.08.087
Sanchez RM, Erhard K, Hardwicke MA, Lin H, McSurdy-Freed J, Plant R, Raha K, Rominger CM, Schaber MD, Spengler MD, Moore ML (2012) Synthesis and structure-activity relationships of 1,2,4-triazolo[1,5-a]pyrimidin-7(3H)-ones as novel series of potent \(\beta \) isoform selective phosphatidylinositol 3-kinase inhibitors. Bioorg Med Chem Lett 22:3198–3202. https://doi.org/10.1016/j.bmcl.2012.03.039
Sutherlin DP, Baker S, Bisconte A, Blaney PM, Brown A, Chan BK, Chantry D, Castanedo G, DePledge P, Goldsmith P, Goldstein DM (2012) Potent and selective inhibitors of \(\text{ PI3K }\delta \): obtaining isoform selectivity from the affinity pocket and tryptophan shelf. Bioorg Med Chem Lett 22:4296–4302. https://doi.org/10.1016/j.bmcl.2012.05.027
Ellard K, Sunose M, Bell K, Ramsden N, Bergamini G, Neubauer G (2012) Discovery of novel PI3K\(\gamma \)/\(\delta \) inhibitors as potential agents for inflammation. Bioorg Med Chem Lett 22:4546–4549. https://doi.org/10.1016/j.bmcl.2012.05.121
Barlaam B, Cosulich S, Degorce S, Fitzek M, Giordanetto F, Green S, Inghardt T, Hennequin L, Hancox U, Lambert-van der Brempt C, Morgentin R (2014) Discovery of 9-(1-anilinoethyl)-2-morpholino-4-oxo-pyrido[1,2-a]pyrimidine-7-carboxamides as PI3K\(\beta \)/\(\delta \) inhibitors for the treatment of PTEN-deficient tumours. Bioorg Med Chem Lett 24:3928–3935. https://doi.org/10.1016/j.bmcl.2014.06.040
Kendall JD, Rewcastle GW, Frederick R, Mawson C, Denny WA, Marshall ES, Baguley BC, Chaussade C, Jackson SP, Shepherd PR (2007) Synthesis, biological evaluation and molecular modelling of sulfonhydrazides as selective PI3K p110alpha inhibitors. Bioorg Med Chem 15:7677–7687. https://doi.org/10.1016/j.bmc.2007.08.062
Kuang RR, Qian F, Li Z, Wei DZJ (2006) Study on improving the selectivity of compounds that inhibit two PI3Ks (gamma and delta). J Mol Model 12:445–452. https://doi.org/10.1007/s00894-005-0069-8
Gonzalez-Lopez de Turiso F, Shin Y, Brown M, Cardozo M, Chen Y, Fong D, Hao X, He X, Henne K, Hu Y, Johnson MG (2012) Discovery and in vivo evaluation of dual PI3K\(\beta \)/\(\delta \) inhibitors. J Med Chem 55:7667–7685. https://doi.org/10.1021/jm300679u
Murray JM, Sweeney ZK, Chan BK, Balazs M, Bradley E, Castanedo G, Chabot C, Chantry D, Flagella M, Goldstein DM, Kondru R (2012) Potent and highly selective benzimidazole inhibitors of PI3-kinase delta. J Med Chem 55:7686–7695. https://doi.org/10.1021/jm300717c
Safina BS, Baker S, Baumgardner M, Blaney PM, Chan BK, Chen YH, Cartwright MW, Castanedo G, Chabot C, Cheguillaume AJ, Goldsmith P (2010) Discovery of novel PI3-kinase \(\delta \) specific inhibitors for the treatment of rheumatoid arthritis: taming CYP3A4 time-dependent inhibition. J Med Chem 55:5887–5900. https://doi.org/10.1021/jm3003747
Discovery Studio Visualization (2012) Release 3.5. Accelrys Inc, San Diego, CA, USA. http://accelrys.com/products/collaborative-science/biovia-discovery-studio/visualization-download.php
Ligprep, version 25, User Manual, Schrödinger, LLC, New York, 2012. https://www.schrodinger.com/ligprep
Rose PW, Beran B, Bi C, Bluhm WF, Dimitropoulos D, Goodsell DS, Prlić A, Quesada M, Quinn GB, Westbrook JD, Young J (2011) The RCSB Protein Data Bank: redesigned web site and web services. Nucleic Acids Res 39:D392–D401. https://doi.org/10.1093/nar/gkq1021
Sastry GM, Adzhigirey M, Day T, Annabhimoju R, Sherman W (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234. https://doi.org/10.1007/s10822-013-9644-8
Glide, version 58, User Manual, Schrödinger, LLC, New York, 2012
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749. https://doi.org/10.1021/jm0306430
Jasuja H, Chadha N, Kaur M, Silakari O (2014) Dual inhibitors of Janus kinase 2 and 3 (JAK2/3): designing by pharmacophore-and docking-based virtual screening approach. Mol Divers 18:253–267. https://doi.org/10.1007/s11030-013-9497-z
Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA (2006) PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening. 1. Methodology and preliminary results. J Comput Aided Mol Des 20:647–671. https://doi.org/10.1007/s10822-006-9087-6
Verma J, Khedkar VM, Coutinho EC (2010) 3D-QSAR in drug design: a review. Curr Top Med Chem 10:95–115. https://doi.org/10.2174/156802610790232260
Golbraikh A, Tropsha A (2002) Beware of q2!. J Mol Graph Model 20:269–276. https://doi.org/10.1016/S1093-3263(01)00123-1
Kaur M, Kumari A, Bahia MS, Silakari O (2013) Designing of new multi-targeted inhibitors of spleen tyrosine kinase (Syk) and zeta-associated protein of 70 kDa (ZAP-70) using hierarchical virtual screening protocol. J Mol Graph Model 39:165–175. https://doi.org/10.1016/j.jmgm.2012.11.011
Weaver S, Gleeson MP (2008) The importance of the domain of applicability in QSAR modeling. J Mol Graph Model 26:1315–1326. https://doi.org/10.1016/j.jmgm.2008.01.002
Mouchlis VD, Melagraki G, Mavromoustakos T, Kollias G, Afantitis A (2012) Molecular modeling on pyrimidine-urea inhibitors of TNF-\(\alpha \) production: an integrated approach using a combination of molecular docking, classification techniques, and 3D-QSAR CoMSIA. J Chem Inf Model 52:711–723. https://doi.org/10.1021/ci200579f
Cramer RD, Bunce JD, Patterson DE, Frank IE (1988) Crossvalidation, bootstrapping, and partial least squares compared with multiple regression in conventional QSAR studies. Quant Struct Act Rel 7:18–25. https://doi.org/10.1002/qsar.19880070105
Salam NK, Nuti R, Sherman W (2009) Novel method for generating structure-based pharmacophores using energetic analysis. J Chem Inf Model 49:2356–2368. https://doi.org/10.1021/ci900212v
Kumar BS, Kotla R, Buddiga R, Roy J, Singh SS, Gundla R, Ravikumar M, Sarma JA (2011) Ligand-based and structure-based approaches in identifying ideal pharmacophore against c-Jun N-terminal kinase-3. J Mol Model 17:151–163. https://doi.org/10.1007/s00894-010-0701-0
Bender A, Glen RC (2005) A discussion of measures of enrichment in virtual screening: comparing the information content of descriptors with increasing levels of sophistication. J Chem Inf Model 45:1369–1375. https://doi.org/10.1021/ci0500177
Lu SH, Wu JW, Liu HL, Zhao JH, Liu KT, Chuang CK, Lin HY, Tsai WB, Ho Y (2011) The discovery of potential acetylcholinesterase inhibitors: a combination of pharmacophore modeling, virtual screening, and molecular docking studies. J Biomed Sci 18:1–13. https://doi.org/10.1186/1423-0127-18-8
QikProp, version 35; Schrödinger, LLC, New York, NY, 2012
Prime, version 31, Schrödinger, LLC, New York, NY, 2012
Desmond Molecular Dynamics System, version 31, D E Shaw Research, New York, NY, 2012
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe SI, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262:5592–5595
Rodems SM, Hamman BD, Lin C, Zhao J, Shah S, Heidary D, Makings L, Stack JH, Pollok BA (2002) A FRET-based assay platform for ultra-high density drug screening of protein kinases and phosphatases. Assay Drug Dev Technol 1:9–19. https://doi.org/10.1089/154065802761001266
Zhang JH, Chung TD, Oldenburg KRJ (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. Biomol Screen 4:67–73. https://doi.org/10.1177/108705719900400206
Csermely P, Agoston V, Pongor S (2005) The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci 26:178–182. https://doi.org/10.1016/j.tips.2005.02.007
Boggon TJ, Li Y, Manley PW, Eck MJ (2005) Crystal structure of the Jak3 kinase domain in complex with a staurosporine analog. Blood 106:996–1002. https://doi.org/10.1182/blood-2005-02-0707
Soth M, Hermann JC, Yee C, Alam M, Barnett JW, Berry P, Browner MF, Frank K, Frauchiger S, Harris S, He Y (2013) 3-Amido pyrrolopyrazine JAK kinase inhibitors: development of a JAK3 vs JAK1 selective inhibitor and evaluation in cellular and in vivo models. J Med Chem 56:345–356. https://doi.org/10.1021/jm301646k
Thoma G, Nuninger F, Falchetto R, Hermes E, Tavares GA, Vangrevelinghe E, Zerwes HG (2011) Identification of a potent Janus Kinase 3 inhibitor with high selectivity within the Janus Kinase family. J Med Chem 54:284–288. https://doi.org/10.1021/jm101157q
Chrencik JE, Patny A, Leung IK, Korniski B, Emmons TL, Hall T, Weinberg RA, Gormley JA, Williams JM, Day JE, Hirsch JL (2010) Structural and thermodynamic characterization of the TYK2 and JAK3 kinase domains in complex with CP-690550 and CMP-6. J Mol Biol 16:413–433. https://doi.org/10.1016/j.jmb.2010.05.020
Bernd AT, Miller S, Williams O (2010) The p110 delta structure: mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol 6:117–124. https://doi.org/10.1038/nchembio.293
Acknowledgements
Authors would like to thank Dr. Ravikumar Muttineni (Application Scientist), Er. Anirban Banerjee (IT Consultant) and Mr. Raghu Rangaswamy from Schrödinger, Bangalore, for their constant scientific and technical support to handle Schrödinger software and work smoothly. Authors also thank Indian Council of Medical Research, New Delhi, for providing the financial support; Grant No. BIC/11(02)/2013. Authors would like to acknowledge Thermo Fisher Scientific, Paisley PA4 9RF, UK, for performing kinase assays.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, M., Singh, P.K., Singh, M. et al. Molecular dynamics and integrated pharmacophore-based identification of dual \(\hbox {JAK3/PI3K}\delta \) inhibitors. Mol Divers 22, 95–112 (2018). https://doi.org/10.1007/s11030-017-9794-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-017-9794-z