Abstract
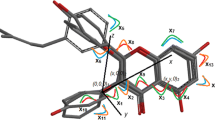
Aromatase, which catalyses the final step in the steroidogenesis pathway of estrogen, has been target for the design of inhibitor in the treatment of hormone dependent breast cancer for postmenopausal women. The extensive SAR studies performed in the last 30 years to search for potent, selective and less toxic compounds, have led to the development of second and third generation of non-steroidal aromatase inhibitors (AI). Besides the development of synthetic compounds, several naturally occurring and synthetic flavonoids, which are ubiquitous natural phenolic compounds and mediate the host of biological activities, are found to demonstrate inhibitory effects on aromatase. The present study explores the pharmacophores, i.e., the structural requirements of flavones (Fig. 1) for inhibition of aromatase activity, using quantitative structure activity relationship (QSAR) and space modeling approaches. The classical QSAR studies generate the model (R 2 = 0.924, Q 2 = 0.895, s = 0.233) that shows the importance of aromatic rings A and C, along with substitutional requirements in meta and para positions of ring C for the activity. 3D QSAR of Comparative Molecular Field Analysis (CoMFA, R 2 = 0.996, \({R^{2}_{cv}=0.791}\)) and Comparative Molecular Similarity Analysis (CoMSIA, R 2 = 0.992, \({R^{2}_{cv}=0.806}\)) studies show contour maps of steric and hydrophobic properties and contribution of acceptor and donor of the molecule, suggesting the presence of steric hindrance due to ring C and R′′-substituent, bulky hydrophobic substitution in ring A, along with acceptors at positions 11, and α and γ of imidazole ring, and donor in ring C favor the inhibitory activity. Further space modeling (CATALYST®) study (R = 0.941, Δ cost = 96.96, rmsd = 0.876) adjudge the presence of hydrogen bond acceptor (keto functional group), hydrophobic (ring A) and aromatic rings (steric hindrance) along with critical distance among features are important for the inhibitory activity.
Similar content being viewed by others
References
Hartmann RW, Ehmer PB, Haidar S et al (2002) Inhibition of CYP 17, a new strategy for the treatment of prostate cancer. Arch Pharm 335: 19–128
Fobes JF (1997) The control of breast cancer: the role of tamoxifene. Semin Oncol 24: S115–S119
Brueggemeier RW (2002) Aromatase inhibitors in breast cancer therapy. Anticancer Ther 2: 181–191
Brueggemeier RW, Richards JA, Joomprabutra S et al (2001) Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J Steroid Biochem Mol Biol 79: 75–84
Banting L, Nicholls PJ, Shaw MA, Smith HJ (1989) Recent developments in aromatase inhibition as a potential treatment for oestrogen-dependent breast cancer. Prog Med Chem 26: 253–298
Pasqualini JR (2004) The selective estrogen enzyme modulators in breast cancer: A review. Biochem Biophys Acta 21: 23–43
Miller WR (2003) Aromatase Inhibitor: mechanism of action and role in the treatment of breast cancer. Semin Oncol 30: 3–11
Howell A (2005) New developments in the treatment of postmenopausal breast cancer. Trends Endocrin Metab 9: 420–428
Carlo DG, Mascolo N, Izzo AA, Capasso F (1999) Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 49: 337–353
Kao YC, Zhou C, Sherman M et al (1998) Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ Health Perspect 106: 85–92
Jeong HJ, Shin YG, Kim IH, Pezzuto JM (1999) Inhibition of aromatase activity by flavonoids. Arch Pharm Res 22: 309–312
Jordan VC, Mittal S, Gosden B et al (1985) Structure–activity relationships of estrogens. Environ Health Perspect 61: 97–110
Le Bail JC, Laroche T, Marre-Fournier F, Habrioux G (1998) Aromatase and 17β-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Let 133: 101–106
Pouget C, Lauthier F, Alain Simon A et al (2001) Flavonoids: structural requirements for antiproliferative activity on breast cancer cells. Bioorg Med Chem Let 11: 3095–3097
Fallowfield L, Cella D, Cuzick J et al (2004) Quality of life of postmenopausal women in the arimidex, tamoxifen, alone or in combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 21: 4261–4271
Montana JG, Dyke HJ (2002) Update on the therapeutic potential of PDE4 inhibitors. Expert Opin Invest Drugs 11: 1–13
Hertog MGL, Feskens EJM, Kromhout D et al (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342: 1007–1011
Kristam R, Gillet VJ, Lewis RA, Thorner D (2005) Comparison of conformational analysis techniques to generate pharmacophore hypotheses using catalyst. J Chem Inf Model 2: 461–476
Guner OF (2000) In pharmacophore perception, development, and use in drug design. International University Line, California
Gobbi S, Cavalli A, Rampa A et al (2006) Lead optimization providing a series of flavone derivatives as potent nonsteroidal inhibitors of the cytochrome P450 aromatase enzyme. J Med Chem 49: 4777–4780
Pouget C, Fagnere C, Basly JP et al (2002) Synthesis and aromatase inhibitory activity of flavanones. Pharma Res 19: 286–291
Snedecor GW, Cochran WG (1967) Statistical methods, 6th edn. Iowa State University Press, Iowa
Wold S, Albano C (1984) Multivariate data analysis in chemistry. In: Kowalski B (ed) CHEMOMETRICS: mathematics and statistics in chemistry, Reidel Dordrecht Netherlands
Hoskuldsson A (1987) PLS regression methods. J Chemometrics 2: 211–228
CS Chem3D Pro and CS MOPAC Pro 5.0, CambridgeSoft, Cambridge. www.camsoft.com
Kier LB, Hall LH (1990) An electrotopological-state index for atoms in molecules. Pharm Res 7: 801–807
ESI-MS (2007) Chemical Technology, Calcutta University, Kolkata. www.caluniv.ac.in
CAChe 6.1, Computer-aided chemistry for macintosh and windows, Fujitsu Systems Business of America Inc. Chiba, Japan. www.CACheSoftware.com
TSAR 3D 3.3, Oxford Molecular Limited, Accelrys, San Diego. www.accelrys.com
Statistica 5.0, StatSoft Inc, Tulsa. www.statsoft.com
Wold S, Eriksson L (1995) Chemometric methods in molecular design. In: de Waterbeemd HV (eds) Chemometric methods, vol 2. Weinheim, VCH Verlagsgesellschaft mbh, p 312
Cramer RD III, Patterson DE, Bunce JD (1989) Recent advances in comparative molecular field analysis (CoMFA). Prog Clin Biol Res 291: 161–165
Allen MS, Tan YC, Koehler KF et al (1990) Synthetic and computer-assisted analyses of the pharmacophore for the benzodiazepine receptor inverse agonist site. J Med Chem 33: 2343–2357
Klebe G, Abraham U, Mietzner T (1994) Molecular similarity indices in a comparative analysis (CoMSIA) of drug molecules to correlate and predict their biological activity. J Med Chem 37: 4130–4146
Cramer RD III, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA). Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110: 5959–5967
Cramer RD III, Bunce JD, Patterson DE et al (1988) Cross-validation, bootstrapping, and partial least squares compared with multiple regression in conventional QSAR and QSPR studies. Quant Struct Act Relat 7: 18–25
Wehrens R, van der Linden WE (1997) Bootstrapping principal component regression models. J Chemom 11: 157–171
SYBYL 7.3, (2006) Tripos Inc, St. Louis. www.tripos.com
Kirkpatrick S, Gelatt CD, Vecchi MP Jr (1983) Optimization by simulated annealing. Science 220: 671–680
Streitwieser A (1961) Molecular orbital theory for organic chemists. Wiley, New York
Vinter JG, Davis A, Saunders MR (1987) Strategic approaches to drug design. I. An integrated software framework for molecular modeling. J Comp-Aided Mol Design 1: 31–51
White DNJ (1977) The principles and practice of molecular mechanics calculations. Comp Chem 1: 225–233
Catalyst 4.11 (2005) Accelrys Inc, San Diego, CA. www.accelrys.com
Schuster D, Laggner C, Steindl TM (2006) Pharmacophore modeling and in-silico screening for new P450 19 (aromatase) inhibitors. J Chem Inf Model 46: 1301–1311
Cavalli A, Bisi A, Bertucci C et al (2005) Enantioselective nonsteroidal aromatase inhibitors identified through a multidisciplinary medicinal chemistry approach. J Med Chem 48: 7282–7289
Recanatini M, Bisi A, Cavalli A et al (2001) A new class of nonsteroidal aromatase inhibitors: Design and synthesis of chromone and xanthone derivatives and inhibition of the P450 enzymes aromatase and 17-hydroxylase/C17,20-lyase. J Med Chem 44: 672–680
Viswanadhan VN, Ghose AK, Revankar GR, Robins RK (1989) Atomic physicochemical parameters for three dimensional structure directed quantitative structure–activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J Chem Inf Comput Sci 29: 163–172
Favia AD, Cavalli A, Masetti M et al (2006) Three dimensional model of the human aromatase enzyme and density functional parameterization of the iron-containing protopophyrin IX for molecular dynamics study of heme-cysteinato cytochromes. Proteins 62: 1074–1087
Gauthier S, Caron B, Cloutier J et al (1997) (S)-(+)-4-[7-(2,2-Dimethyl-1-oxopropoxy)-4-methyl-2-[4-[2-(1-piperidinyl)- ethoxy]phenyl]-2H-1-benzopyran-3-yl]-phenyl-2,2-dimethylpropanoate (EM-800): a highly potent, specific, and orally active nonsteroidal antiestrogen. J Med Chem 40: 2117–2122
Miller CP, Collini MD, Tran BD et al (2001) Design, synthesis, and preclinical characterization of novel, highly selective indole estrogens. J Med Chem 44: 1654–1657
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagar, S., Islam, M.A., Das, S. et al. Pharmacophore mapping of flavone derivatives for aromatase inhibition. Mol Divers 12, 65–76 (2008). https://doi.org/10.1007/s11030-008-9077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-008-9077-9