Abstract
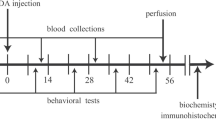
Human studies indicate that Parkinson’s disease (PD) associates with disruption in metabolism of glucose and free fatty acids (FFA). Studies have shown that interlukin-1beta (IL-1β) causes hypoglycemia through insulin- independent mechanisms. Here, we investigated association between dopaminergic neuronal death, as the main pathophysiological mechanism underlying PD, and serum levels of glucose, FFA and IL-1β in 6-hydroxydopamine (6-OHDA) animal model of PD. Neurotoxin of 6-OHDA was injected into medial forebrain bundle and multiple behavioral testes were carried out during eight weeks thereafter. Blood was collected before the toxin and in second and eight weeks thereafter. Then, brain of the animals was perfused to assess survival of dopaminergic (DAergic) neurons in substantia nigra by tyrosine hydroxylase (TH) immunohistochemistry. Glucose, FFA and IL-1β levels were determined using calorimetric method and specific ELISA kits. In compare to control, 6-OHDA- treated rats had less glucose and FFA levels in the eight week and higher IL-1β level in the both second and eight weeks. Based on severity of behavioral symptoms, 6-OHDA- treated rats were divided into two subgroups of severe and mild. Number of TH- positive cells in these subgroups was 83 and 45% less than that in control. Also, both subgroups showed less weight gain, lower glucose and FFA and higher IL-1β in eight week. Our data indicate that moderate to severe progressive DAergic neuronal death in substantia nigra associates with a decrease in serum levels of glucose and FFA. Increase in IL-1β production following neuronal death possibly mediated this decrease.





Similar content being viewed by others
Data availability statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Change history
26 September 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11011-023-01298-0
References
Adani G, Filippini T, Michalke B, Vinceti M (2020) Selenium and Other Trace Elements in the Etiology of Parkinson’s disease: A Systematic Review and Meta-Analysis of Case-Control Studies. Neuroepidemiology 54:1–23
Alrafiah A, Al-Ofi E, Obaid MT, Alsomali N (2019) Assessment of the Levels of Level of Biomarkers of Bone Matrix Glycoproteins and Inflammatory Cytokines from Saudi Parkinson Patients. Biomedical Research International 2019:2690205
Barbeau A, Sourkes TL (1961) Some biochemical aspects of extrapyramidal diseases. Rev Can Biol 20:197–203
Batisse-Lignier M, Rieu I, Guillet C, Pujos E, Morio B, Lemaire JJ, …. BoirieY, (2013) Deep brain stimulation of the subthalamic nucleus regulates postabsorptive glucose metabolism in patients with Parkinson’s disease. J Clin Endocrinol Metab 98(6):E1050–E1054
Berkenbosch F, de Goeij DE, Rey AD, Besedovsky HO (1989) Neuroendocrine, sympathetic and metabolic responses induced by interleukin-1. Neuroendocrinology 50(5):570–576
Bird TA, Davies A, Baldwin SA, Saklatvala J (1990) Interleukin 1 stimulates hexose transport in fibroblasts by increasing the expression of glucose transporters. J Biol Chem 65(23):13578–13583
Bowen BC, Block RE, Sanchez-Ramos J, Pattany PM, Lampman DA, Murdoch JB, Quencer RM (1995) Proton MR Spectroscopy of the brain in 14 patients with Parkinson disease. Am J Neuroradiol 16(1):61–68
Chan YK, Davis PF, Poppitt SD, Sun X, Greenhill NS, KrishnamurthiR,….Krissansen G, (2012) Influence of tail versus cardiac sampling on blood glucose and lipid profiles in mice. Lab Anim 46(2):142–147
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474
Crotty S, Fitzgerald P, Tuohy E, Harris DM, Fishe A, Mandel A, …. Nolan Y, (2008) Neuroprotective effects of novel phosphatidylglycerol-based phospholipids in the 6-hydroxydopamine model of Parkinson’s disease. Eur J Neurosci 27(2):294–300
De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT (2017) Association of Autonomic Dysfunction With Disease Progression and Survival in Parkinson Disease. JAMA Neurol 74(8):970–976
Del Rey A, Monge-Arditi G, Besedovsky HO (1998) Central and peripheral mechanisms contribute to the hypoglycemia induced by interleukin-1. (1998). Annals of New York Academy of Science 840:153–161
Esmaeili MH, Movahedi M, Faraji A, Haghdoost-Yazdi H (2012) Intracerebral injection of low amounts of norharman induces moderate Parkinsonism-like behavioral symptoms in rat. Neurotoxicology Teratology 34:489–494
Firbank MJ, Yarnall AJ, Lawson RA, Duncan GW, Khoo TK, Petrides GS, …, Burn DJ (2017) Cerebral glucose metabolism and cognition in newly diagnosed Parkinson’s disease: ICICLE-PD study. Journal of Neurology Neurosurgery and Psychiatry 88(4): 310-316
Fujita M, Nishino H, Kumazaki M, Shimada S, Tohyama M, Nishimura T (1996) Expression of dopamine transporter mRNA and its binding site in fetal nigral cells transplanted into the striatum of 6-OHDA lesioned rat. Mol Brain Res 39:127–136
Garcia-Welsh A, Schneiderman JS, Baly DL (1990) Interleukin-1 stimulates glucose transport in rat adipose cells. Evidence for receptor discrimination between IL-1 beta and IL-1 alpha. FEBS Letter 269(2):421–4
Henry J (1991) Clinical Diagnosis and Management by Laboratory Methods. WB Saunders, Philadelphia
Hu MT, Chaudhuri KR, Jarosz J, Yaguez L, Brooks DJ (2002) An imaging study of Parkinsonism among African-Caribbean and Indian London communities. Mov Disord 17(6):1321–1328
Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debre P, …. Hirsch EC, (1999) FcεRII/CD23 is expressed in Parkinson’s disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-α in glial cells. J Neurosci 19:3440–3447
Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162:1–10
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Karpenko MN, Vasilishina AA, Gromova EA, Muruzheva ZM, Miliukhina IV, Bernadotte A (2018) Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-alpha levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell Immunology 327:77–82
Koprich JB, Reske-Nielsen C, Mithal P, Isacson O (2008) Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation 5:8
Leal MC, Casabona JC, Puntel M, Pitossi F (2013) Interleukin-1β and tumor necrosis factor-α: reliable targets for protective therapies in Parkinson’s disease? Front Cell Neurosci 7:53
Luo S, Meier AH, Cincotta AH (1998) Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters. Neuroendocrinology 68(1):1–10
Marques A, Dutheil F, Durand E, Rieu I, Mulliez A, Fantini ML, …. Durif F, (2018) Glucose dysregulation in Parkinson’s disease: Too much glucose or not enough insulin? Parkinsonism Relat Disord 55:122–127
Minaei A, Haghdoost-Yazdi H (2019) Dexmedetomidine attenuates the induction and reverses the progress of 6-hydroxydopamine-induced Parkinsonism; involvement of KATP channels, alpha 2 adrenoceptors and anti-inflammatory mechanisms. Toxicology and applied pharmacology 382:114743
Minaei A, Sarookhani MR, Haghdoost-Yazdi H, Rajaei F (2021) Hydrogen sulfide attenuates induction and prevents progress of the 6-hydroxydopamine-induced Parkinsonism in rat through activation of ATP-sensitive potassium channels and suppression of ER stress. Toxicol Appl Pharmacol 423:115558. https://doi.org/10.1016/j.taap.2021.115558
Mittelman SD, Bergman RN (2000) Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab 279(3):E630–E637
Ota K, Wildmann J, Ota T, Besedovsky HO, Del Rey A (2009) Interleukin-1beta and insulin elicit different neuroendocrine responses to hypoglycemia. Annals of New York Academy of Science 1153:82–88
Palmiter RD (2007) Is dopamine a physiologically relevant mediator of feeding behavior? Trends in Neuroscience 30(8):375–381
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego, CA
Rebrin K, Steil GM, Mittelman SD, Bergman RN (1996) Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. The Journal of Clinical Investment 98(3):741–749
Ristow M (2004) Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (berl) 82(8):510–529
Rodrigues SF, de Oliveira MA, Martins JO, Sannomiya P, de Cássia TR, Nigro D, Fortes ZB (2006) Differential effects of chloral hydrate- and ketamine/xylazine-induced anesthesia by the s.c. route. Life Science 79:1630–1637
Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA (2005) Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Experimental Biology and Medicine (Maywood, N.J.) 230:777–84
Sandyk R (1993) The relationship between diabetes mellitus and Parkinson’s disease. Int J Neurosci 69(1–4):125–130
Santiago JA, Potashkin JA (2013) Integrative network analysis unveils convergent molecular pathways in Parkinson’s disease and diabetes. PLoS One 8(12):e83940
Sarukhani MR, Haghdoost-Yazdi H, Khandan-Chelarci G (2018) Changes in the Serum Urate Level Can Predict the Development of Parkinsonism in the 6- Hydroxydopamine Animal Model. Neurochem Res 43(5):1086–1095
Schallert T, Kozlowski DA, Humm JL, Cocke RR (1997) Use-dependent structural events in recovery of function. Advances in Neurology 73:229–38
Schulte EC, Altmaier E, Berger HS, Do KT, Kastenmüller G, Wahl S, …. Winkelmann J (2016) Alterations in Lipid and Inositol Metabolisms in Two Dopaminergic Disorders. PLoS One 11(1): e0147129
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222
Trupp M, Jonsson P, Ohrfelt A, Zetterberg H, Obudulu O, Malm L, Wuolikainen A, …Forsgren L (2014) Metabolite and peptide levels in plasma and CSF differentiating healthy controls from patients with newly diagnosed Parkinson’s disease. Journal of Parkinson’s disease 4(3): 549-60
Wang W, Meng X, Yang C, Fang D, Wang X, An J, … Gao Y (2017) Brown adipose tissue activation in a rat model of Parkinson’s disease. American Journal of Physiology, Endocrinology and Metabolism 313(6): E731-E736
Wang W, Tan M, Yu J, Tan L (2015) Role of pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease 3:1–15
Wang Z, Yang Y, Xiang X, Zhu Y, Men J, He M, … Jiu Y (2010) Estimation of the normal range of blood glucose in rats. Journal of hygiene research 39(2): 133-7
Willems JL, Buylaert WA, Lefebvre RA, Bogaert MG (1985) Neuronal dopamine receptors on autonomic ganglia and sympathetic nerves and dopamine receptors in the gastrointestinal system. Pharmacology Review 37(2):165–216
Wise RA (2006) Role of brain dopamine in food reward and reinforcement. Philosophical Transactions of the Royal Society b: Biological Sciences 361(1471):1149–1158
Xie L, Hu LF, Teo XQ, Tiong CX, Tazzari V, Sparatore A, …. Bian JS (2013) Therapeutic effect of hydrogen sulfide-releasing L-Dopa derivative ACS84 on 6-OHDA-induced Parkinson’s disease rat model. PLoS One 8: e60200
Yuan H, Sarre S, Ebinger G, Michotte Y (2005) Histological, behavioral and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods 144(1):35–45
Zhang FL, He Y, Zheng Y, Zhang WJ, Wang Q, Jia YJ, …Wang XM, (2014) Therapeutic effects of fucoidan in 6-hydroxydopamine- lesioned rat model of Parkinson’s disease: Role of NADPH oxidase-1. CNS Neuroscience Therapy 20(12):1036–1044
Acknowledgements
We would like to thank ladies Nafiseh Rastgoo and Azita Minaei for their assistance in behavioral tests, blood collections and IHC assessments.
Author information
Authors and Affiliations
Contributions
Study was designed by Haghdoost-Yazdi, H and Sarbazi-Golezari A. Both authors involved in performing the experiments. Data was analyzed by Sarbazi-Golezari A and manuscript wrote by both authors.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures of the present study were carried out according to the guidelines of animal experiments of the Research Council at Qazvin University of Medical Sciences. This study was supported by a grant-in-aid for scientific research from the Research Council of Qazvin University of Medical Sciences (grant number: IR.QUMS.REC.1397.320).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11011-023-01298-0
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarbazi-Golezari, A., Haghdoost-Yazdi, H. RETRACTED ARTICLE: Chronic and progressive dopaminergic neuronal death in substantia nigra associates with a decrease in serum levels of glucose and free fatty acids, the role of interlokin-1 beta. Metab Brain Dis 37, 373–381 (2022). https://doi.org/10.1007/s11011-021-00868-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00868-4