Abstract
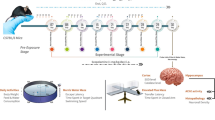
Alzheimer’s disease (AD) is a multifactorial disorder where amyloid beta (Aβ) plaques, Ca2+ dysregulation, excessive oxidative stress, mitochondrial dysfunction and synaptic loss operate synergistically to bring about cholinergic deficits and dementia. New therapeutic interventions are gaining prominence as the morbidity and mortality of AD increases exponentially every year. Treating AD with antihypertensive drugs is thought to be a promising intervention; however, its mechanism of action of ameliorating AD needs further investigation. In this context, the present study explores the protective effect of verapamil, an antihypertensive agent of Ca2+ channel blocker (CCB) class against scopolamine-induced in vitro neurotoxicity and in vivo cognitive impairment. Supplementation of verapamil was found to attenuate oxidative stress by preventing mitochondrial injury, and augment the expression of genes involved in the cholinergic function (mACR1), synaptic plasticity (GAP43, SYP) and Ca2+-dependent memory-related genes (CREB1, CREBBP, BDNF). Further, verapamil treatment in mice attenuated the cognitive and behavioural deficits induced by scopolamine as measured by the elevated plus maze and passive avoidance test (P < 0.05). Thus, the present study demonstrates the neuroprotective effect of verapamil against the pathogenesis of AD such as oxidative stress, mitochondrial dysfunction and cognitive decline. These observations emphasize the importance of ‛Ca2+ dysregulation’ and ‛mitochondrial dysfunction’ theories in AD and recommends the supplementation of compounds that regulate Ca2+ homeostasis and mitochondrial function in susceptible AD individuals.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Abbreviations
- Aβ:
-
Amyloid beta
- DMEM:
-
Dulbecco’s modified eagle medium
- DMSO:
-
Dimethyl sulfoxide
- DNPH :
-
2,4-dinitrophenylhydrazine
- DTNB :
-
5,5′-dithiobis-(2-nitrobenzoic acid)
- EDTA :
-
Ethylenediaminetetraacetic acid
- FBS :
-
Fetal bovine serum
- GAPDH :
-
Glyceraldehyde 3-phosphate dehydrogenase
- HBSS :
-
Hank’s balanced salt solution
- Iso-OMPA :
-
tetraisopropyl pyrophosphoramide
- JC1 :
-
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
- LTP :
-
Long term potentiation
- MTT :
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NBT :
-
Nitroblue tretazolium
- PBS :
-
Phosphate buffered saline
- VOC :
-
Voltage operated channel
References
Balon R, Ramesh C (1996) Calcium channel blockers for anxiety disorders? Ann Clin Psychiatry 8:215–220
Brodie C, Sampson SR (1991) Verapamil regulation of Na-K pump levels in rat skeletal myotubes: role of spontaneous activity and Na channels. J Neurosci Res 28:229–235. https://doi.org/10.1002/jnr.490280210
Chen G, Zou X, Watanabe H et al (2010) CREB binding protein is required for both short-term and long-term memory formation. J Neurosci 30:13066–13077. https://doi.org/10.1523/JNEUROSCI.2378-10.2010
Chinopoulos C, Adam-Vizi V (2006) Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J 273:433–450. https://doi.org/10.1111/j.1742-4658.2005.05103.x
Chintoh A, Fulton J, Koziel N et al (2003) Role of cholinergic receptors in locomotion induced by scopolamine and oxotremorine-M. Pharmacol Biochem Behav 76:53–61
Darwish I, Dessouky I (2015) Potential Neuroprotective Role of Verapamil in Experimentally- Induced Chronic Sciatic Nerve Constriction in Mice. Br J Med Med Res 8:781–789. https://doi.org/10.9734/BJMMR/2015/17908
Di Carlo M, Giacomazza D, Picone P et al (2012) Are oxidative stress and mitochondrial dysfunction the key players in the neurodegenerative diseases? Free Radic Res 46:1327–1338. https://doi.org/10.3109/10715762.2012.714466
Diadiushka GP (1979) Inhibitory effect of verapamil on the acetylcholinesterase activity of skeletal muscle sarcolemma. Biokhimiia 44:1912–1917
Dickey CA, Loring JF, Montgomery J et al (2003) Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J Neurosci 23(12):5219–5226. https://doi.org/10.1523/JNEUROSCI.23-12-05219.2003
Dubovsky SL, Franks RD, Allen S, Murphy J (1986) Calcium antagonists in mania: a double-blind study of verapamil. Psychiatry Res 18:309–320
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Finger S, Green L, Tarnoff ME et al (1990) Nimodipine enhances new learning after hippocampal damage. Exp Neurol 109:279–285
Fisher A, Pittel Z, Haring R et al (2003) M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer’s disease: implications in future therapy. J Mol Neurosci 20:349–356. https://doi.org/10.1385/JMN:20:3:349
Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI (1995) Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer’s disease. J Neurochem 64:1888–1891
Freir DB, Costello DA, Herron CE (2003) A beta 25-35-induced depression of long-term potentiation in area CA1 in vivo and in vitro is attenuated by verapamil. J Neurophysiol 89:3061–3069. https://doi.org/10.1152/jn.00992.2002
Fulga IG, Stroescu V (1997) Experimental research on the effect of calcium channel blockers nifedipine and verapamil on the anxiety in mice. Rom J Physiol 34:127–136
Gurtu S, Seth S, Roychoudhary AK (1992) Evidence for verapamil-induced functional inhibition of noradrenergic neurotransmission in vivo. Naunyn Schmiedeberg's Arch Pharmacol 345:172–175
Haile M, Limson F, Gingrich K et al (2009) Nimodipine prevents transient cognitive dysfunction after moderate hypoxia in adult mice. J Neurosurg Anesthesiol 21:140–144. https://doi.org/10.1097/ANA.0b013e3181920d28
Höschl C (1991) Do calcium antagonists have a place in the treatment of mood disorders? Drugs 42:721–729. https://doi.org/10.2165/00003495-199142050-00001
Höschl C, Vacková J, Janda B (1992) Mood stabilizing effect of verapamil. Bratisl Lek Listy 93:208–209
Hsieh M-T, Hsieh C-L, Lin L-W et al (2003) Differential gene expression of scopolamine-treated rat hippocampus-application of cDNA microarray technology. Life Sci 73:1007–1016
Ingole SR, Satyendra KR, Sharma SS (2008) Cognition Enhancers: Current Strategies and Future Perspectives. CRIPS 9:42–48
Kalonia H, Kumar P, Kumar A (2011) Attenuation of proinflammatory cytokines and apoptotic process by verapamil and diltiazem against quinolinic acid induced Huntington like alterations in rats. Brain Res 1372:115–126. https://doi.org/10.1016/j.brainres.2010.11.060
Kedziora-Kornatowska K, Szram S, Kornatowski T et al (2002) The effect of verapamil on the antioxidant defence system in diabetic kidney. Clin Chim Acta 322:105–112
Kelley SR, Kamal TJ, Molitch ME (1996) Mechanism of verapamil calcium channel blockade-induced hyperprolactinemia. Am J Physiol 270:E96–100. https://doi.org/10.1152/ajpendo.1996.270.1.E96
Konar A, Shah N, Singh R et al (2011) Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One 6:e27265. https://doi.org/10.1371/journal.pone.0027265
Konrad T, Beier K, Kusterer K et al (1997) The effect of verapamil on mitochondrial calcium content in normoxic, hypoxic and reoxygenated rat liver. Histochem J 29:309–315
Koo WS, Gengaro PE, Burke TJ, Schrier RW (1995) Verapamil attenuates calcium-induced mitochondrial swelling and respiratory dysfunction. J Pharmacol Exp Ther 273:206–212
Kwon S-H, Lee H-K, Kim J-A et al (2010) Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol 649:210–217. https://doi.org/10.1016/j.ejphar.2010.09.001
Lakhina V, Arey RN, Kaletsky R et al (2015) Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron 85:330–345. https://doi.org/10.1016/j.neuron.2014.12.029
Lebois EP, Bridges TM, Lewis LM et al (2010) Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M(1) receptor function in the central nervous system. ACS Chem Neurosci 1:104–121. https://doi.org/10.1021/cn900003h
Lee EH, Lin WR (1991) Nifedipine and verapamil block the memory-facilitating effect of corticotropin-releasing factor in rats. Life Sci 48:1333–1340
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Li N, Liu G (2010) The novel squamosamide derivative FLZ enhances BDNF/TrkB/CREB signaling and inhibits neuronal apoptosis in APP/PS1 mice. Acta Pharmacol Sin 31:265–272. https://doi.org/10.1038/aps.2010.3
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. https://doi.org/10.1038/nature05292
Liu Y, Lo Y-C, Qian L et al (2011) Verapamil protects dopaminergic neuron damage through a novel anti-inflammatory mechanism by inhibition of microglial activation. Neuropharmacology 60:373–380. https://doi.org/10.1016/j.neuropharm.2010.10.002
López-Arrieta JM, Birks J (2002) Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev CD000147. doi: https://doi.org/10.1002/14651858.CD000147
Lovell MA, Abner E, Kryscio R et al (2015) Calcium Channel Blockers, Progression to Dementia, and Effects on Amyloid Beta Peptide Production. Oxidative Med Cell Longev 2015:787805. https://doi.org/10.1155/2015/787805
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mak IT, Weglicki WB (1990) Comparative antioxidant activities of propranolol, nifedipine, verapamil, and diltiazem against sarcolemmal membrane lipid peroxidation. Circ Res 66:1449–1452
Maniskas ME, Roberts JM, Aron I et al (2016) Stroke neuroprotection revisited: Intra-arterial verapamil is profoundly neuroprotective in experimental acute ischemic stroke. J Cereb Blood Flow Metab 36:721–730. https://doi.org/10.1177/0271678X15608395
Matesic DF, Lin RC (1994) Microtubule-associated protein 2 as an early indicator of ischemia-induced neurodegeneration in the gerbil forebrain. J Neurochem 63:1012–1020
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
O’Brien FE, O’Connor RM, Clarke G et al (2013) P-glycoprotein inhibition increases the brain distribution and antidepressant-like activity of escitalopram in rodents. Neuropsychopharmacology 38:2209–2219. https://doi.org/10.1038/npp.2013.120
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Palit G, Kalsotra A, Kumar R et al (2001) Behavioural and anti-psychotic effects of Ca2+ channel blockers in rhesus monkey. Eur J Pharmacol 412:139–144
Pandareesh MD, Anand T (2013) Neuromodulatory propensity of Bacopa monniera against scopolamine-induced cytotoxicity in PC12 cells via down-regulation of AChE and up-regulation of BDNF and muscarnic-1 receptor expression. Cell Mol Neurobiol 33:875–884. https://doi.org/10.1007/s10571-013-9952-5
Pandya JD, Nukala VN, Sullivan PG (2013) Concentration dependent effect of calcium on brain mitochondrial bioenergetics and oxidative stress parameters. Front Neuroenerg 5:10. https://doi.org/10.3389/fnene.2013.00010
Pauwels PJ, Van Assouw HP, Peeters L, Leysen JE (1990) Neurotoxic action of veratridine in rat brain neuronal cultures: mechanism of neuroprotection by Ca++ antagonists nonselective for slow Ca++ channels. J Pharmacol Exp Ther 255:1117–1122
Peng T-I, Jou M-J (2010) Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci 1201:183–188. https://doi.org/10.1111/j.1749-6632.2010.05634.x
Phillips HS, Hains JM, Armanini M et al (1991) BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 7:695–702
Poorheidari G, Pratt JA, Dehghani N (2002) Effects of low-dose scopolamine on locomotor activity: No dissociation between cognitive and non-effects. Neurosci Res Commun 31:165–174. https://doi.org/10.1002/nrc.10049
Popović M, Caballero-Bleda M, Popović N et al (1997a) Neuroprotective effect of chronic verapamil treatment on cognitive and noncognitive deficits in an experimental Alzheimer’s disease in rats. Int J Neurosci 92:79–93
Popović M, Caballero-Bleda M, Popović N et al (2006) Verapamil prevents, in a dose-dependent way, the loss of ChAT-immunoreactive neurons in the cerebral cortex following lesions of the rat nucleus basalis magnocellularis. Exp Brain Res 170:368–375. https://doi.org/10.1007/s00221-005-0219-3
Popović M, Popović N, Jovanova-Nesić K et al (1997b) Effect of physostigmine and verapamil on active avoidance in an experimental model of Alzheimer’s disease. Int J Neurosci 90:87–97
Pucilowski O (1992) Psychopharmacological properties of calcium channel inhibitors. Psychopharmacology 109:12–29
Pugazhenthi S, Wang M, Pham S et al (2011) Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener 6:60. https://doi.org/10.1186/1750-1326-6-60
Quartermain D, deSoria VG, Kwan A (2001) Calcium channel antagonists enhance retention of passive avoidance and maze learning in mice. Neurobiol Learn Mem 75:77–90. https://doi.org/10.1006/nlme.1999.3958
Quartermain D, Garcia de Soria V (2001) The effects of calcium channel antagonists on short- and long-term retention in mice using spontaneous alternation behavior. Neurobiol Learn Mem 76:117–124. https://doi.org/10.1006/nlme.2000.3981
Rauer H, Grissmer S (1999) The effect of deep pore mutations on the action of phenylalkylamines on the Kv1.3 potassium channel. Br J Pharmacol 127:1065–1074. https://doi.org/10.1038/sj.bjp.0702599
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 14:45–53. https://doi.org/10.1016/j.molmed.2007.12.002
Reddy PH, Mani G, Park BS et al (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis 7:103–117 discussion 173-180
Ressler KJ, Nemeroff CB (2000) Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety 12(Suppl 1):2–19. https://doi.org/10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4
Saravanaraman P, Chinnadurai RK, Boopathy R (2014) Why calcium channel blockers could be an elite choice in the treatment of Alzheimer’s disease: a comprehensive review of evidences. Rev Neurosci 25:231–246. https://doi.org/10.1515/revneuro-2013-0056
Scott Bitner R (2012) Cyclic AMP response element-binding protein (CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer’s disease therapeutics. Biochem Pharmacol 83:705–714. https://doi.org/10.1016/j.bcp.2011.11.009
Shirey JK, Brady AE, Jones PJ et al (2009) A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci 29:14271–14286. https://doi.org/10.1523/JNEUROSCI.3930-09.2009
Siegel HN, Lukas RJ (1986) Allosteric modification of alpha-bungarotoxin binding by the “calcium channel antagonist” verapamil. Brain Res 387:37–42
Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu Rev Neurosci 21:127–148. https://doi.org/10.1146/annurev.neuro.21.1.127
Sitges M, Reyes A (1995) Effects of verapamil on the release of different neurotransmitters. J Neurosci Res 40:613–621. https://doi.org/10.1002/jnr.490400506
Soltani MH, Pichardo R, Song Z et al (2005) Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. Am J Pathol 166:1841–1850. https://doi.org/10.1016/S0002-9440(10)62493-5
Srere PA, Brazil H, Gonen L, Takahashi M (1963) The Citrate Condensing Enzyme of Pigeon Breast Muscle and Moth Flight Muscle. Acta Chem Scand (17 supl):129–134. https://doi.org/10.3891/acta.chem.scand.17s-0129
Taya K, Watanabe Y, Kobayashi H, Fujiwara M (2000) Nimodipine improves the disruption of spatial cognition induced by cerebral ischemia. Physiol Behav 70:19–25
Trofimiuk E, Holownia A, Braszko JJ (2010) Activation of CREB by St. John’s wort may diminish deletorious effects of aging on spatial memory. Arch Pharm Res 33:469–477. https://doi.org/10.1007/s12272-010-0318-y
Tsuda K, Tsuda S, Goldstein M, Masuyama Y (1993) Effects of verapamil and diltiazem on dopamine release in the central nervous system of spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 20:641–645
Umukoro S, Ashorobi R, Essein E (2006) Anticonvulsant and Anxiolytic Effects of Calcium Channel Blockers in Mice. J Med Sci (Faisalabad) 6:1021–1024. https://doi.org/10.3923/jms.2006.1021.1024
Villard V, Espallergues J, Keller E et al (2011) Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (σ1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J Psychopharmacol (Oxford) 25:1101–1117. https://doi.org/10.1177/0269881110379286
Waite M, Van Deenen LL, Ruigrok TJ, Elbers PF (1969) Relation of mitochondrial phospholipase A activity to mitochondrial swelling. J Lipid Res 10:599–608
Wu D, Lu J, Zheng Y et al (2008) Purple sweet potato color repairs d-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol Learn Mem 90:19–27. https://doi.org/10.1016/j.nlm.2008.01.010
Xiao J, Li S, Sui Y et al (2014) Lactobacillus casei-01 facilitates the ameliorative effects of proanthocyanidins extracted from lotus seedpod on learning and memory impairment in scopolamine-induced amnesia mice. PLoS One 9:e112773. https://doi.org/10.1371/journal.pone.0112773
Yamada K, Nabeshima T (2003) Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci 91:267–270
Zhang W, Wang GM, Wang PJ et al (2014) Effects of neural stem cells on synaptic proteins and memory in a mouse model of Alzheimer’s disease. J Neurosci Res 92:185–194. https://doi.org/10.1002/jnr.23299
Acknowledgements
We acknowledge the support from Dr. G. Ariharasivakumar (KMCH College of Pharmacy, Coimbatore, India) and R. Vadivelan (JSS College of Pharmacy, Ooty, India) for animal studies. The authors PS and RKC respectively acknowledge the research fellowships DST-INSPIRE and DST-PURSE, both provided by the Department of Science and Technology, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that no conflict of interest exists with respect to the authorship and publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ponne, S., Kumar, C.R. & Boopathy, R. Verapamil attenuates scopolamine induced cognitive deficits by averting oxidative stress and mitochondrial injury – A potential therapeutic agent for Alzheimer’s Disease. Metab Brain Dis 35, 503–515 (2020). https://doi.org/10.1007/s11011-019-00498-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00498-x