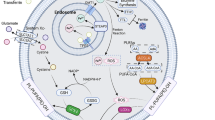
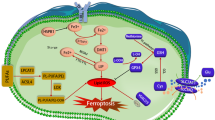
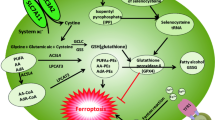
Abstract
Ferroptosis is a newly identified form of programmed cell death that is connected to iron-dependent lipid peroxidization. It involves a variety of physiological processes involving iron metabolism, lipid metabolism, oxidative stress, and biosynthesis of nicotinamide adenine dinucleotide phosphate, glutathione, and coenzyme Q10. So far, it has been discovered to contribute to the pathological process of many diseases, such as myocardial infarction, acute kidney injury, atherosclerosis, and so on. Macrophages are innate immune system cells that regulate metabolism, phagocytize pathogens and dead cells, mediate inflammatory reactions, promote tissue repair, etc. Emerging evidence shows strong associations between macrophages and ferroptosis, which can provide us with a deeper comprehension of the pathological process of diseases and new targets for the treatments. In this review, we summarized the crosstalk between macrophages and ferroptosis and anatomized the application of this association in disease treatments, both non-neoplastic and neoplastic diseases. In addition, we have also addressed problems that remain to be investigated, in the hope of inspiring novel therapeutic strategies for diseases.





Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1–2):317–331. https://doi.org/10.1016/j.cell.2013.12.010
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379. https://doi.org/10.1038/cdd.2015.158
Yang WS, Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15(3):234–245. https://doi.org/10.1016/j.chembiol.2008.02.010
Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R et al (2021) Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res 28:231–243. https://doi.org/10.1016/j.jare.2020.07.007
Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C et al (2021) ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun 93:312–321. https://doi.org/10.1016/j.bbi.2021.01.003
Fang J, Yuan Q, Du Z, Zhang Q, Yang L, Wang M et al (2023) Overexpression of GPX4 attenuates cognitive dysfunction through inhibiting hippocampus ferroptosis and neuroinflammation after traumatic brain injury. Free Radic Biol Med 204:68–81. https://doi.org/10.1016/j.freeradbiomed.2023.04.014
Bai T, Li M, Liu Y, Qiao Z, Wang Z (2020) Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic Biol Med 160:92–102. https://doi.org/10.1016/j.freeradbiomed.2020.07.026
Su G, Yang W, Wang S, Geng C, Guan X (2021) SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem Biophys Res Commun 561:33–39. https://doi.org/10.1016/j.bbrc.2021.05.011
Ouyang S, You J, Zhi C, Li P, Lin X, Tan X et al (2021) Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis 12(8):782. https://doi.org/10.1038/s41419-021-04054-3
Yu W, Liu W, Xie D, Wang Q, Xu C, Zhao H et al (2022) High level of uric acid promotes atherosclerosis by targeting NRF2-mediated autophagy dysfunction and ferroptosis. Oxid Med Cell Longev. https://doi.org/10.1155/2022/9304383
Xu LC, Cao J, Li WJ, Yang ZM, Zhao R, Zhang JR et al (2022) Ferroptosis in laryngeal squamous cell carcinoma and its regulation by M2 macrophage-derived exosomes. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 57(3):324–332. https://doi.org/10.3760/cma.j.cn115330-20210621-00361
Li H, Yang P, Wang J, Zhang J, Ma Q, Jiang Y et al (2022) HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol 15(1):2. https://doi.org/10.1186/s13045-021-01223-x
Dai E, Han L, Liu J, Xie Y, Zeh HJ, Kang R et al (2020) Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun 11(1):6339. https://doi.org/10.1038/s41467-020-20154-8
Kremer DM, Nelson BS, Lin L, Yarosz EL, Halbrook CJ, Kerk SA et al (2021) GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun 12(1):4860. https://doi.org/10.1038/s41467-021-24859-2
Hao X, Zheng Z, Liu H, Zhang Y, Kang J, Kong X et al (2022) Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol 56:102463. https://doi.org/10.1016/j.redox.2022.102463
Tang B, Xu W, Wang Y, Zhu J, Wang H, Tu J et al (2021) Identification of critical ferroptosis regulators in lung adenocarcinoma that RRM2 facilitates tumor immune infiltration by inhibiting ferroptotic death. Clin Immunol 232:108872. https://doi.org/10.1016/j.clim.2021.108872
Liu T, Zhu C, Chen X, Guan G, Zou C, Shen S et al (2022) Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro-Oncology 24(7):1113–1125. https://doi.org/10.1093/neuonc/noac033
Alborzinia H, Flórez AF, Kreth S, Brückner LM, Yildiz U, Gartlgruber M et al (2022) MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat Cancer 3(4):471–485. https://doi.org/10.1038/s43018-022-00355-4
Amaral EP, Costa DL, Namasivayam S, Riteau N, Kamenyeva O, Mittereder L et al (2019) A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J Exp Med 216(3):556–570. https://doi.org/10.1084/jem.20181776
He R, Liu B, Xiong R, Geng B, Meng H, Lin W et al (2022) Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov 8(1):43. https://doi.org/10.1038/s41420-021-00807-3
Wu J, Liu Q, Zhang X, Tan M, Li X, Liu P et al (2022) The interaction between STING and NCOA4 exacerbates lethal sepsis by orchestrating ferroptosis and inflammatory responses in macrophages. Cell Death Dis 13(7):653. https://doi.org/10.1038/s41419-022-05115-x
Ma H, Dong Y, Chu Y, Guo Y, Li L (2022) The mechanisms of ferroptosis and its role in alzheimer’s disease. Front Mol Biosci 9:965064. https://doi.org/10.3389/fmolb.2022.965064
Dang Y, He Q, Yang S, Sun H, Liu Y, Li W et al (2022) FTH1- and SAT1-induced astrocytic ferroptosis is involved in Alzheimer’s disease: evidence from single-cell transcriptomic analysis. Pharmaceuticals (Basel). https://doi.org/10.3390/ph15101177
Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X et al (2019) DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med 131:356–369. https://doi.org/10.1016/j.freeradbiomed.2018.12.011
Sabatier M, Birsen R, Lauture L, Mouche S, Angelino P, Dehairs J et al (2023) C/EBPα confers dependence to fatty acid anabolic pathways and vulnerability to lipid oxidative stress-induced ferroptosis in FLT3-mutant leukemia. Cancer Discov 13(7):1720–1747. https://doi.org/10.1158/2159-8290.Cd-22-0411
Liang C, Zhang X, Yang M, Dong X (2019) Recent progress in ferroptosis inducers for cancer therapy. Adv Mater 31(51):e1904197. https://doi.org/10.1002/adma.201904197
Bashir S, Sharma Y, Elahi A, Khan F (2016) Macrophage polarization: the link between inflammation and related diseases. Inflamm Res 65(1):1–11. https://doi.org/10.1007/s00011-015-0874-1
Chen X, Liu Y, Gao Y, Shou S, Chai Y (2021) The roles of macrophage polarization in the host immune response to sepsis. Int Immunopharmacol 96:107791. https://doi.org/10.1016/j.intimp.2021.107791
Nelson BN, Hawkins AN, Wozniak KL (2020) Pulmonary macrophage and dendritic cell responses to cryptococcus neoformans. Front Cell Infect Microbiol 10:37. https://doi.org/10.3389/fcimb.2020.00037
Woo YD, Jeong D, Chung DH (2021) Development and functions of alveolar macrophages. Mol Cells 44(5):292–300. https://doi.org/10.14348/molcells.2021.0058
Borst K, Dumas AA, Prinz M (2021) Microglia: immune and non-immune functions. Immunity 54(10):2194–2208. https://doi.org/10.1016/j.immuni.2021.09.014
Nayak D, Roth TL, McGavern DB (2014) Microglia development and function. Annu Rev Immunol 32:367–402. https://doi.org/10.1146/annurev-immunol-032713-120240
Kwon HS, Koh SH (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9(1):42. https://doi.org/10.1186/s40035-020-00221-2
Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN (2017) Mechanisms of foam cell formation in atherosclerosis. J Mol Med (Berl) 95(11):1153–1165. https://doi.org/10.1007/s00109-017-1575-8
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L (2019) Macrophages and metabolism in the tumor microenvironment. Cell Metab 30(1):36–50. https://doi.org/10.1016/j.cmet.2019.06.001
Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V et al (2020) Tumor-associated macrophages in human breast, colorectal, lung ovarian and prostate cancers. Front Oncol 10:566511. https://doi.org/10.3389/fonc.2020.566511
Zhao L, Zhou X, Xie F, Zhang L, Yan H, Huang J et al (2022) Ferroptosis in cancer and cancer immunotherapy. Cancer Commun (Lond) 42(2):88–116. https://doi.org/10.1002/cac2.12250
Mbah NE, Lyssiotis CA (2022) Metabolic regulation of ferroptosis in the tumor microenvironment. J Biol Chem 298(3):101617. https://doi.org/10.1016/j.jbc.2022.101617
Yu H, Yang C, Jian L, Guo S, Chen R, Li K et al (2019) Sulfasalazine-induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol Rep 42(2):826–838. https://doi.org/10.3892/or.2019.7189
Kajarabille N, Latunde-Dada GO (2019) Programmed cell-death by ferroptosis: antioxidants as mitigators. Int J Mol Sci. https://doi.org/10.3390/ijms20194968
Cui Y, Zhang Z, Zhou X, Zhao Z, Zhao R, Xu X et al (2021) Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J Neuroinflam 18(1):249. https://doi.org/10.1186/s12974-021-02231-x
Dar HH, Anthonymuthu TS, Ponomareva LA, Souryavong AB, Shurin GV, Kapralov AO et al (2021) A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(·) sabotage of theft-ferroptosis. Redox Biol 45:102045. https://doi.org/10.1016/j.redox.2021.102045
Gu Z, Liu T, Liu C, Yang Y, Tang J, Song H et al (2021) Ferroptosis-strengthened metabolic and inflammatory regulation of tumor-associated macrophages provokes potent tumoricidal activities. Nano Lett 21(15):6471–6479. https://doi.org/10.1021/acs.nanolett.1c01401
Perrotta C, Cervia D, Di Renzo I, Moscheni C, Bassi MT, Campana L et al (2018) Nitric oxide generated by tumor-associated macrophages is responsible for cancer resistance to cisplatin and correlated with syntaxin 4 and acid sphingomyelinase inhibition. Front Immunol 9:1186. https://doi.org/10.3389/fimmu.2018.01186
Tamura R, Tanaka T, Yamamoto Y, Akasaki Y, Sasaki H (2018) Dual role of macrophage in tumor immunity. Immunotherapy 10(10):899–909. https://doi.org/10.2217/imt-2018-0006
Prenen H, Mazzone M (2019) Tumor-associated macrophages: a short compendium. Cell Mol Life Sci 76(8):1447–1458. https://doi.org/10.1007/s00018-018-2997-3
Klöditz K, Fadeel B (2019) Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov 5:65. https://doi.org/10.1038/s41420-019-0146-x
Fadeel B, Xue D (2009) The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit Rev Biochem Mol Biol 44(5):264–277. https://doi.org/10.1080/10409230903193307
Tyurin VA, Balasubramanian K, Winnica D, Tyurina YY, Vikulina AS, He RR et al (2014) Oxidatively modified phosphatidylserines on the surface of apoptotic cells are essential phagocytic ‘eat-me’ signals: cleavage and inhibition of phagocytosis by Lp-PLA2. Cell Death Differ 21(5):825–835. https://doi.org/10.1038/cdd.2014.1
Wang Y, Subramanian M, Yurdagul A Jr, Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J et al (2017) Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell 171(2):331-345.e22. https://doi.org/10.1016/j.cell.2017.08.041
Vago JP, Amaral FA, van de Loo FAJ (2021) Resolving inflammation by TAM receptor activation. Pharmacol Ther 227:107893. https://doi.org/10.1016/j.pharmthera.2021.107893
Kourtzelis I, Li X, Mitroulis I, Grosser D, Kajikawa T, Wang B et al (2019) DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat Immunol 20(1):40–49. https://doi.org/10.1038/s41590-018-0249-1
Mehrotra P, Ravichandran KS (2022) Drugging the efferocytosis process: concepts and opportunities. Nat Rev Drug Discov 21(8):601–620. https://doi.org/10.1038/s41573-022-00470-y
Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Le Manh T et al (2019) Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun 10(1):2091. https://doi.org/10.1038/s41467-019-09753-2
Nagata S (2018) Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 36:489–517. https://doi.org/10.1146/annurev-immunol-042617-053010
Yang Z, Li Q, Wang X, Jiang X, Zhao D, Lin X et al (2018) C-type lectin receptor LSECtin-mediated apoptotic cell clearance by macrophages directs intestinal repair in experimental colitis. Proc Natl Acad Sci USA 115(43):11054–11059. https://doi.org/10.1073/pnas.1804094115
Peter C, Waibel M, Keppeler H, Lehmann R, Xu G, Halama A et al (2012) Release of lysophospholipid ‘find-me’ signals during apoptosis requires the ATP-binding cassette transporter A1. Autoimmunity 45(8):568–573. https://doi.org/10.3109/08916934.2012.719947
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461(7261):282–286. https://doi.org/10.1038/nature08296
Zhang H, Guo Z, Guo Y, Wang Z, Tang Y, Song T et al (2021) Bim transfer between Bcl-2-like protein and Hsp70 underlines Bcl-2/Hsp70 crosstalk to regulate apoptosis. Biochem Pharmacol 190:114660. https://doi.org/10.1016/j.bcp.2021.114660
Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA et al (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498(7454):371–375. https://doi.org/10.1038/nature12175
Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF et al (2010) Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci USA 107(8):3546–3551. https://doi.org/10.1073/pnas.0914351107
Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN (2019) Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 177(3):541–55.e17. https://doi.org/10.1016/j.cell.2019.02.028
Westman J, Grinstein S, Marques PE (2019) Phagocytosis of necrotic debris at sites of injury and inflammation. Front Immunol 10:3030. https://doi.org/10.3389/fimmu.2019.03030
Liu N, Liang Y, Wei T, Zou L, Huang X, Kong L et al (2022) The role of ferroptosis mediated by NRF2/ERK-regulated ferritinophagy in CdTe QDs-induced inflammation in macrophage. J Hazard Mater 436:129043. https://doi.org/10.1016/j.jhazmat.2022.129043
Zhao X, Si L, Bian J, Pan C, Guo W, Qin P et al (2022) Adipose tissue macrophage-derived exosomes induce ferroptosis via glutathione synthesis inhibition by targeting SLC7A11 in obesity-induced cardiac injury. Free Radic Biol Med 182:232–245. https://doi.org/10.1016/j.freeradbiomed.2022.02.033
Liu J, Zhu S, Zeng L, Li J, Klionsky DJ, Kroemer G et al (2022) DCN released from ferroptotic cells ignites AGER-dependent immune responses. Autophagy 18(9):2036–2049. https://doi.org/10.1080/15548627.2021.2008692
Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ et al (2021) Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ 28(6):1971–1789. https://doi.org/10.1038/s41418-020-00719-2
Wen Q, Liu J, Kang R, Zhou B, Tang D (2019) The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun 510(2):278–283. https://doi.org/10.1016/j.bbrc.2019.01.090
Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J et al (2021) Author correction: selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol 22(12):1599. https://doi.org/10.1038/s41590-021-01063-4
Wang Q, Imamura R, Motani K, Kushiyama H, Nagata S, Suda T (2013) Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int Immunol 25(6):363–372. https://doi.org/10.1093/intimm/dxs161
Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV et al (2020) Succination inactivates gasdermin D and blocks pyroptosis. Science 369(6511):1633–1637. https://doi.org/10.1126/science.abb9818
Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y et al (2020) Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. https://doi.org/10.1126/science.aaz7548
Wang Y, Gao W, Shi X, Ding J, Liu W, He H et al (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547(7661):99–103. https://doi.org/10.1038/nature22393
Elliott MR, Ravichandran KS (2016) The dynamics of apoptotic cell clearance. Dev Cell 38(2):147–160. https://doi.org/10.1016/j.devcel.2016.06.029
Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L et al (2010) High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem 285(51):39888–39897. https://doi.org/10.1074/jbc.M110.128348
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L et al (2014) HMGB1 in health and disease. Mol Aspects Med 40:1–116. https://doi.org/10.1016/j.mam.2014.05.001
Yu Y, Xie Y, Cao L, Yang L, Yang M, Lotze MT et al (2015) The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol 2(4):e1054549. https://doi.org/10.1080/23723556.2015.1054549
Kang R, Tang D (2017) Autophagy and ferroptosis—what’s the connection? Curr Pathobiol Rep 5(2):153–159. https://doi.org/10.1007/s40139-017-0139-5
Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR et al (2014) The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene 33(5):567–577. https://doi.org/10.1038/onc.2012.631
Parameswaran N, Patial S (2010) Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 20(2):87–103. https://doi.org/10.1615/critreveukargeneexpr.v20.i2.10
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL et al (2018) Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol 29(3):919–935. https://doi.org/10.1681/asn.2017050523
Djudjaj S, Martin IV, Buhl EM, Nothofer NJ, Leng L, Piecychna M et al (2017) Macrophage migration inhibitory factor limits renal inflammation and fibrosis by counteracting tubular cell cycle arrest. J Am Soc Nephrol 28(12):3590–3604. https://doi.org/10.1681/asn.2017020190
Atri C, Guerfali FZ, Laouini D (2018) Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. https://doi.org/10.3390/ijms19061801
Guerrero-Hue M, García-Caballero C, Palomino-Antolín A, Rubio-Navarro A, Vázquez-Carballo C, Herencia C et al (2019) Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. Faseb J 33(8):8961–8975. https://doi.org/10.1096/fj.201900077R
Zhou L, Xue X, Hou Q, Dai C (2022) Targeting ferroptosis attenuates interstitial inflammation and kidney fibrosis. Kidney Dis (Basel) 8(1):57–71. https://doi.org/10.1159/000517723
Wang Y, Chen Z, Luo J, Zhang J, Sang AM, Cheng ZS et al (2023) Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. Int Immunopharmacol 115:109731. https://doi.org/10.1016/j.intimp.2023.109731
Friedmann Angeli JP, Krysko DV, Conrad M (2019) Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer 19(7):405–414. https://doi.org/10.1038/s41568-019-0149-1
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90. https://doi.org/10.1038/nchembio.2238
D’Herde K, Krysko DV (2017) Ferroptosis: oxidized PEs trigger death. Nat Chem Biol 13(1):4–5. https://doi.org/10.1038/nchembio.2261
Murray PJ (2017) Macrophage polarization. Annu Rev Physiol 79:541–566. https://doi.org/10.1146/annurev-physiol-022516-034339
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41(1):14–20. https://doi.org/10.1016/j.immuni.2014.06.008
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32(5):593–604. https://doi.org/10.1016/j.immuni.2010.05.007
Ganz T (2012) Macrophages and systemic iron homeostasis. J Innate Immun 4(5–6):446–453. https://doi.org/10.1159/000336423
Xia Y, Li Y, Wu X, Zhang Q, Chen S, Ma X et al (2021) Ironing out the details: how iron orchestrates macrophage polarization. Front Immunol 12:669566. https://doi.org/10.3389/fimmu.2021.669566
Mesquita G, Silva T, Gomes AC, Oliveira PF, Alves MG, Fernandes R et al (2020) H-Ferritin is essential for macrophages’ capacity to store or detoxify exogenously added iron. Sci Rep 10(1):3061. https://doi.org/10.1038/s41598-020-59898-0
Jung M, Mertens C, Brüne B (2015) Macrophage iron homeostasis and polarization in the context of cancer. Immunobiology 220(2):295–304. https://doi.org/10.1016/j.imbio.2014.09.011
Recalcati S, Locati M, Gammella E, Invernizzi P, Cairo G (2012) Iron levels in polarized macrophages: regulation of immunity and autoimmunity. Autoimmun Rev 11(12):883–889. https://doi.org/10.1016/j.autrev.2012.03.003
Mu Q, Chen L, Gao X, Shen S, Sheng W, Min J et al (2021) The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci Bull 66(17):1806–1816. https://doi.org/10.1016/j.scib.2021.02.010
Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX, You Y et al (2018) Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med 7(8):4012–4022. https://doi.org/10.1002/cam4.1670
Gan ZS, Wang QQ, Li JH, Wang XL, Wang YZ, Du HH (2017) Iron reduces M1 macrophage polarization in RAW264.7 macrophages associated with inhibition of STAT1. Mediators Inflamm 2017:8570818. https://doi.org/10.1155/2017/8570818
Costa da Silva M, Breckwoldt MO, Vinchi F, Correia MP, Stojanovic A, Thielmann CM et al (2017) Iron induces anti-tumor activity in tumor-associated macrophages. Front Immunol 8:1479. https://doi.org/10.3389/fimmu.2017.01479
Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A et al (2016) Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11(11):986–994. https://doi.org/10.1038/nnano.2016.168
Hu ZW, Wen YH, Ma RQ, Chen L, Zeng XL, Wen WP et al (2021) Ferroptosis driver SOCS1 and suppressor FTH1 independently correlate with M1 and M2 macrophage infiltration in head and neck squamous cell carcinoma. Front Cell Dev Biol 9:727762. https://doi.org/10.3389/fcell.2021.727762
Handa P, Thomas S, Morgan-Stevenson V, Maliken BD, Gochanour E, Boukhar S et al (2019) Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J Leukoc Biol 105(5):1015–1026. https://doi.org/10.1002/jlb.3a0318-108r
Hu X, Cai X, Ma R, Fu W, Zhang C, Du X (2019) Iron-load exacerbates the severity of atherosclerosis via inducing inflammation and enhancing the glycolysis in macrophages. J Cell Physiol 234(10):18792–18800. https://doi.org/10.1002/jcp.28518
Yuan Y, Chen Y, Peng T, Li L, Zhu W, Liu F et al (2019) Mitochondrial ROS-induced lysosomal dysfunction impairs autophagic flux and contributes to M1 macrophage polarization in a diabetic condition. Clin Sci (Lond) 133(15):1759–1777. https://doi.org/10.1042/cs20190672
Zhang B, Yang Y, Yi J, Zhao Z, Ye R (2021) Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J Periodontal Res 56(5):991–1005. https://doi.org/10.1111/jre.12912
Sun S, Wu Y, Maimaitijiang A, Huang Q, Chen Q (2022) Ferroptotic cardiomyocyte-derived exosomes promote cardiac macrophage M1 polarization during myocardial infarction. PeerJ 10:e13717. https://doi.org/10.7717/peerj.13717
Cosin-Roger J, Ortiz-Masià MD, Barrachina MD (2019) Macrophages as an emerging source of Wnt ligands: relevance in mucosal integrity. Front Immunol 10:2297. https://doi.org/10.3389/fimmu.2019.02297
Yuan C, Yang D, Ma J, Yang J, Xue J, Song F et al (2020) Modulation of Wnt/β-catenin signaling in IL-17A-mediated macrophage polarization of RAW264.7 cells. Braz J Med Biol Res 53(8):e9488. https://doi.org/10.1590/1414-431x20209488
Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ et al (2020) Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 16(11):2069–2083. https://doi.org/10.1080/15548627.2020.1714209
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C et al (2018) JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab 27(1):136–50.e5. https://doi.org/10.1016/j.cmet.2017.11.001
Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B (2020) Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol 36:101670. https://doi.org/10.1016/j.redox.2020.101670
Pan Y, Li J, Wang J, Jiang Q, Yang J, Dou H et al (2022) Ferroptotic MSCs protect mice against sepsis via promoting macrophage efferocytosis. Cell Death Dis 13(9):825. https://doi.org/10.1038/s41419-022-05264-z
Xu Y, Liu X, Li Y, Dou H, Liang H, Hou Y (2021) SPION-MSCs enhance therapeutic efficacy in sepsis by regulating MSC-expressed TRAF1-dependent macrophage polarization. Stem Cell Res Ther 12(1):531. https://doi.org/10.1186/s13287-021-02593-2
Jung M, Weigert A, Mertens C, Rehwald C, Brüne B (2017) Iron handling in tumor-associated macrophages-is there a new role for lipocalin-2? Front Immunol 8:1171. https://doi.org/10.3389/fimmu.2017.01171
Lei G, Zhuang L, Gan B (2022) Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer 22(7):381–396. https://doi.org/10.1038/s41568-022-00459-0
Zhao YY, Lian JX, Lan Z, Zou KL, Wang WM, Yu GT (2023) Ferroptosis promotes anti-tumor immune response by inducing immunogenic exposure in HNSCC. Oral Dis 29(3):933–941. https://doi.org/10.1111/odi.14077
Liu J, Dai E, Kang R, Kroemer G, Tang D (2021) The dark side of ferroptosis in pancreatic cancer. Oncoimmunology 10(1):1868691. https://doi.org/10.1080/2162402x.2020.1868691
Boone BA, Murthy P, Miller-Ocuin J, Doerfler WR, Ellis JT, Liang X et al (2018) Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Cancer 18(1):678. https://doi.org/10.1186/s12885-018-4584-2
Shi L, Liu Y, Li M, Luo Z (2022) Emerging roles of ferroptosis in the tumor immune landscape: from danger signals to anti-tumor immunity. Febs J 289(13):3655–3665. https://doi.org/10.1111/febs.16034
Zhang F, Li F, Lu GH, Nie W, Zhang L, Lv Y et al (2019) Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS Nano 13(5):5662–5673. https://doi.org/10.1021/acsnano.9b00892
Ruiz-de-Angulo A, Bilbao-Asensio M, Cronin J, Evans SJ, Clift MJD, Llop J et al (2020) Chemically programmed vaccines: iron catalysis in nanoparticles enhances combination immunotherapy and immunotherapy-promoted tumor ferroptosis. iScience 23(9):101499. https://doi.org/10.1016/j.isci.2020.101499
Wu S, Yang J, Sun G, Hu J, Zhang Q, Cai J et al (2021) Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br J Pharmacol 178(18):3783–3796. https://doi.org/10.1111/bph.15518
Okubo K, Kurosawa M, Kamiya M, Urano Y, Suzuki A, Yamamoto K et al (2018) Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat Med 24(2):232–238. https://doi.org/10.1038/nm.4462
Chen J, Fu CY, Shen G, Wang J, Xu L, Li H et al (2022) Macrophages induce cardiomyocyte ferroptosis via mitochondrial transfer. Free Radic Biol Med 190:1–14. https://doi.org/10.1016/j.freeradbiomed.2022.07.015
Wu J, Feng Z, Chen L, Li Y, Bian H, Geng J et al (2022) TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun 13(1):676. https://doi.org/10.1038/s41467-021-27948-4
Giannini D, Antonucci M, Petrelli F, Bilia S, Alunno A, Puxeddu I (2020) One year in review 2020: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 38(3):387–397
Phull AR, Nasir B, Haq IU, Kim SJ (2018) Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact 281:121–136. https://doi.org/10.1016/j.cbi.2017.12.024
Udalova IA, Mantovani A, Feldmann M (2016) Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol 12(8):472–485. https://doi.org/10.1038/nrrheum.2016.91
Kalliolias GD, Ivashkiv LB (2016) TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 12(1):49–62. https://doi.org/10.1038/nrrheum.2015.169
McInnes IB, Schett G (2017) Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389(10086):2328–2337. https://doi.org/10.1016/s0140-6736(17)31472-1
Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R et al (2020) Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol 16(3):278–290. https://doi.org/10.1038/s41589-019-0462-8
Xiang DM, Sun W, Ning BF, Zhou TF, Li XF, Zhong W et al (2018) The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut 67(9):1704–1715. https://doi.org/10.1136/gutjnl-2016-313392
Weng YS, Tseng HY, Chen YA, Shen PC, Al Haq AT, Chen LM et al (2019) MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer 18(1):42. https://doi.org/10.1186/s12943-019-0988-0
Gao R, Kalathur RKR, Coto-Llerena M, Ercan C, Buechel D, Shuang S et al (2021) YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med 13(12):e14351. https://doi.org/10.15252/emmm.202114351
Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D et al (2020) CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 19(1):43. https://doi.org/10.1186/s12943-020-01168-8
Jiang Q, Wang K, Zhang X, Ouyang B, Liu H, Pang Z et al (2020) Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small 16(22):e2001704. https://doi.org/10.1002/smll.202001704
Kroll AV, Fang RH, Zhang L (2017) Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjug Chem 28(1):23–32. https://doi.org/10.1021/acs.bioconjchem.6b00569
Rao L, Bu LL, Ma L, Wang W, Liu H, Wan D et al (2018) Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew Chem Int Ed Engl 57(4):986–991. https://doi.org/10.1002/anie.201709457
Shen L, Zhou Y, He H, Chen W, Lenahan C, Li X et al (2021) Crosstalk between macrophages, T cells, and iron metabolism in tumor microenvironment. Oxid Med Cell Longev 2021:8865791. https://doi.org/10.1155/2021/8865791
Wang Y, Yu L, Ding J, Chen Y (2018) Iron metabolism in cancer. Int J Mol Sci. https://doi.org/10.3390/ijms20010095
Nielsen SR, Schmid MC (2017) Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm 2017:9624760. https://doi.org/10.1155/2017/9624760
Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG et al (2010) Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70(19):7465–7475. https://doi.org/10.1158/0008-5472.Can-10-1439
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN et al (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545(7655):495–499. https://doi.org/10.1038/nature22396
Sang Y, Deng Q, Cao F, Liu Z, You Y, Liu H et al (2021) Remodeling macrophages by an iron nanotrap for tumor growth suppression. ACS Nano 15(12):19298–19309. https://doi.org/10.1021/acsnano.1c05392
Yin Y, Yao S, Hu Y, Feng Y, Li M, Bian Z et al (2017) The immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clin Cancer Res 23(23):7375–7387. https://doi.org/10.1158/1078-0432.Ccr-17-1283
Gimbrone MA Jr, García-Cardeña G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118(4):620–636. https://doi.org/10.1161/circresaha.115.306301
Fernández-García V, González-Ramos S, Avendaño-Ortiz J, Martín-Sanz P, Delgado C, Castrillo A et al (2022) NOD1 splenic activation confers ferroptosis protection and reduces macrophage recruitment under pro-atherogenic conditions. Biomed Pharmacother 148:112769. https://doi.org/10.1016/j.biopha.2022.112769
Martinet W, Coornaert I, Puylaert P, De Meyer GRY (2019) Macrophage death as a pharmacological target in atherosclerosis. Front Pharmacol 10:306. https://doi.org/10.3389/fphar.2019.00306
Li M, Xin S, Gu R, Zheng L, Hu J, Zhang R et al (2022) Novel diagnostic biomarkers related to oxidative stress and macrophage ferroptosis in atherosclerosis. Oxid Med Cell Longev 2022:8917947. https://doi.org/10.1155/2022/8917947
Liu T, Bao R, Wang Q, Hao W, Liu Y, Chang S et al (2022) SiO(2)-induced ferroptosis in macrophages promotes the development of pulmonary fibrosis in silicosis models. Toxicol Res (Camb) 11(1):42–51. https://doi.org/10.1093/toxres/tfab105
Wu YT, Zhong LS, Huang C, Guo YY, Jin FJ, Hu YZ et al (2022) β-Caryophyllene acts as a ferroptosis inhibitor to ameliorate experimental colitis. Int J Mol Sci. https://doi.org/10.3390/ijms232416055
Ryter SW, Alam J, Choi AM (2006) Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86(2):583–650. https://doi.org/10.1152/physrev.00011.2005
Wegiel B, Otterbein LE (2012) Go green: the anti-inflammatory effects of biliverdin reductase. Front Pharmacol 3:47. https://doi.org/10.3389/fphar.2012.00047
Campbell NK, Fitzgerald HK, Dunne A (2021) Regulation of inflammation by the antioxidant haem oxygenase 1. Nat Rev Immunol 21(7):411–425. https://doi.org/10.1038/s41577-020-00491-x
Ma C, Wu X, Zhang X, Liu X, Deng G (2022) Heme oxygenase-1 modulates ferroptosis by fine-tuning levels of intracellular iron and reactive oxygen species of macrophages in response to Bacillus Calmette-Guerin infection. Front Cell Infect Microbiol 12:1004148. https://doi.org/10.3389/fcimb.2022.1004148
Chinta KC, Rahman MA, Saini V, Glasgow JN, Reddy VP, Lever JM et al (2018) Microanatomic distribution of myeloid heme oxygenase-1 protects against free radical-mediated immunopathology in human tuberculosis. Cell Rep 25(7):1938–52.e5. https://doi.org/10.1016/j.celrep.2018.10.073
Yang S, Ouyang J, Lu Y, Harypursat V, Chen Y (2022) A dual role of heme oxygenase-1 in tuberculosis. Front Immunol 13:842858. https://doi.org/10.3389/fimmu.2022.842858
Wu Y, Wu B, Zhang Z, Lu H, Fan C, Qi Q et al (2020) Heme protects intestinal mucosal barrier in DSS-induced colitis through regulating macrophage polarization in both HO-1-dependent and HO-1-independent way. Faseb J 34(6):8028–8043. https://doi.org/10.1096/fj.202000313RR
Campbell NK, Williams DG, Fitzgerald HK, Barry PJ, Cunningham CC, Nolan DP et al (2019) Trypanosoma brucei secreted aromatic ketoacids activate the Nrf2/HO-1 pathway and suppress pro-inflammatory responses in primary murine glia and macrophages. Front Immunol 10:2137. https://doi.org/10.3389/fimmu.2019.02137
Campbell NK, Fitzgerald HK, Fletcher JM, Dunne A (2019) Plant-derived polyphenols modulate human dendritic cell metabolism and immune function via AMPK-dependent induction of heme oxygenase-1. Front Immunol 10:345. https://doi.org/10.3389/fimmu.2019.00345
Shimizu J, Murao A, Nofi C, Wang P, Aziz M (2022) Extracellular CIRP promotes GPX4-mediated ferroptosis in sepsis. Front Immunol 13:903859. https://doi.org/10.3389/fimmu.2022.903859
Qu C, Dai E, Lai T, Cao G, Liu J, Kang R et al (2021) Itaconic acid induces ferroptosis by activating ferritinophagy. Biochem Biophys Res Commun 583:56–62. https://doi.org/10.1016/j.bbrc.2021.10.054
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530. https://doi.org/10.1038/mp.2017.171
Hirata Y, Yamada C, Ito Y, Yamamoto S, Nagase H, Oh-Hashi K et al (2018) Novel oxindole derivatives prevent oxidative stress-induced cell death in mouse hippocampal HT22 cells. Neuropharmacology 135:242–252. https://doi.org/10.1016/j.neuropharm.2018.03.015
Zhang Q, Qu Y, Zhang Q, Li F, Li B, Li Z et al (2022) Exosomes derived from hepatitis B virus-infected hepatocytes promote liver fibrosis via miR-222/TFRC axis. Cell Biol Toxicol. https://doi.org/10.1007/s10565-021-09684-z
Hu Z, Yin Y, Jiang J, Yan C, Wang Y, Wang D et al (2022) Exosomal miR-142-3p secreted by hepatitis B virus (HBV)-hepatocellular carcinoma (HCC) cells promotes ferroptosis of M1-type macrophages through SLC3A2 and the mechanism of HCC progression. J Gastrointest Oncol 13(2):754–767. https://doi.org/10.21037/jgo-21-916
Ito F, Kato K, Yanatori I, Murohara T, Toyokuni S (2021) Ferroptosis-dependent extracellular vesicles from macrophage contribute to asbestos-induced mesothelial carcinogenesis through loading ferritin. Redox Biol 47:102174. https://doi.org/10.1016/j.redox.2021.102174
Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsky-Moshe S, Lifshitz L et al (2018) Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 131(3):342–352. https://doi.org/10.1182/blood-2017-02-768580
Ito F, Yanatori I, Maeda Y, Nimura K, Ito S, Hirayama T et al (2020) Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and β-catenin induction in mesothelium. Redox Biol 36:101616. https://doi.org/10.1016/j.redox.2020.101616
Liu N, Liang Y, Wei T, Zou L, Bai C, Huang X et al (2022) Protein corona mitigated the cytotoxicity of CdTe QDs to macrophages by targeting mitochondria. NanoImpact 25:100367. https://doi.org/10.1016/j.impact.2021.100367
Kelley SM, Ravichandran KS (2021) Putting the brakes on phagocytosis: “don’t-eat-me” signaling in physiology and disease. EMBO Rep 22(6):e52564. https://doi.org/10.15252/embr.202152564
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW et al (2019) CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572(7769):392–396. https://doi.org/10.1038/s41586-019-1456-0
Acknowledgements
There is no financial conflict of interest between the authors. The authors would like to acknowledge the support of fundings. The authors would like to thank graphics program Adobe Illustrator and smart.servier.com for supporting.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 82001037), the Natural Science Foundation of Sichuan Province (Grant No. 2022NSFSC0751) and the Research and Develop Program, West China Hospital of Stomatology, Sichuan University (Grant No. RD-02-202007) for Xuelian Tan.
Author information
Authors and Affiliations
Contributions
XT conceived and designed the study. WL drafted the manuscript. LY polished the language. WL, LY and XT searched and reviewed the literature, and created the figures and tables. All of the authors critically reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, W., Yang, L. & Tan, X. Crosstalk between ferroptosis and macrophages: potential value for targeted treatment in diseases. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04871-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04871-4