Abstract
Drug addiction is a devastating condition that poses a serious burden on the society. The use of some drugs like morphine for their tremendous analgesic properties is also accompanied with developing tolerance, dependence and the withdrawal symptoms. These symptoms are frequently severe enough to reinforce the person in recovery to start over the use of drug again and hinder the clinical use of drugs like morphine for chronic pain. Research into opioid receptors and related molecular pathways has seen resurgence in the wake of the growing opioid epidemic. The current study provides a comprehensive scientific exploration of the molecular mechanisms and underlying signalling in morphine tolerance and dependence. It also critically evaluates current therapeutic approaches, shedding light on their efficacy and limitations, and future prospects.
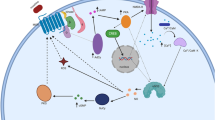
Graphical Abstract
The graphical abstract depicts an overview of the pathways involved in the emergence of morphine-related tolerance and dependence including NMDA, Nitric oxide, and PPAR, as well as behavioural sensitization along with present and future innovative treatment strategies including stem cell therapy that have been discussed in the current manuscript.


Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Krishnamurti C, Rao SC (2016) The isolation of morphine by Serturner. Indian J Anaesth 60:861
Su L-Y, Liu Q, Jiao L, Yao Y-G (2021) Molecular mechanism of neuroprotective effect of melatonin on morphine addiction and analgesic tolerance: an update. Mol Neurobiol 58:4628–4638
Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703
Kreek M, Reed B, Butelman E (2019) Current status of opioid addiction treatment and related preclinical research. Sci Adv 5:eaax9140
Spencer MR, Warner M, Bastian BA, Trinidad JP, Hedegaard H (2019) Drug overdose deaths involving fentanyl, 2011–2016. Natl Vital Stat Rep 68:1–19
Hedegaard H, Bastian BA, Trinidad JP, Spencer M and Warner M (2018) Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. National Center for Health Statistics (U.S.);United States. Food and Drug. https://stacks.cdc.gov/view/cdc/61381
Khan MI, Momeny M, Ostadhadi S, Jahanabadi S, Ejtemaei-Mehr S, Sameem B, Zarrinrad G, Dehpour AR (2018) Thalidomide attenuates development of morphine dependence in mice by inhibiting PI3K/Akt and nitric oxide signaling pathways. Prog Neuropsychopharmacol Biol Psychiatry 82:39–48
Javadi S, Ejtemaeimehr S, Keyvanfar HR, Moghaddas P, Aminian A, Rajabzadeh A, Mani AR, Dehpour AR (2013) Pioglitazone potentiates development of morphine-dependence in mice: possible role of NO/cGMP pathway. Brain Res 1510:22–37
Harris AC, Gewirtz JC (2005) Acute opioid dependence: characterizing the early adaptations underlying drug withdrawal. Psychopharmacology 178:353–366
Evans CJ, Cahill CM (2016) Neurobiology of opioid dependence in creating addiction vulnerability. F1000Research 5:1748
Listos J, Baranowska-Bosiacka I, Talarek S, Listos P, Orzelska J, Fidecka S, Gutowska I, Kolasa A, Rybicka M, Chlubek D (2013) The effect of perinatal lead exposure on dopamine receptor D2 expression in morphine dependent rats. Toxicology 310:73–83
Nicoara D, Zhang Y, Nelson JT, Brewer AL, Maharaj P, DeWald SN, Shirachi DY, Quock RM (2016) Hyperbaric oxygen treatment suppresses withdrawal signs in morphine-dependent mice. Brain Res 1648:434–437
Zebraski SE, Kochenash SM, Raffa RB (2000) Lung opioid receptors: pharmacology and possible target for nebulized morphine in dyspnea. Life Sci 66:2221–2231
Little JW, Cuzzocrea S, Bryant L, Esposito E, Doyle T, Rausaria S, Neumann WL, Salvemini D (2013) Spinal mitochondrial-derived peroxynitrite enhances neuroimmune activation during morphine hyperalgesia and antinociceptive tolerance. PAIN® 154:978–986
Khan MI, Ostadhadi S, Mumtaz F, Momeny M, Moghaddaskho F, Hassanipour M, Ejtemaei-Mehr S, Dehpour AR (2017) Thalidomide attenuates the development and expression of antinociceptive tolerance to μ-opioid agonist morphine through l-arginine-iNOS and nitric oxide pathway. Biomed Pharmacother 85:493–502
Mitra S, Sinatra RS, Warltier DC (2004) Perioperative management of acute pain in the opioid-dependent patient. J Am Soc Anesthesiol 101:212–227
Hassanipour M, Amini-Khoei H, Shafaroodi H, Shirzadian A, Rahimi N, Imran-Khan M, Rezayat S-M, Dehpour A (2016) Atorvastatin attenuates the antinociceptive tolerance of morphine via nitric oxide dependent pathway in male mice. Brain Res Bull 125:173–180
Kotlinska J, Gibula-Bruzda E, Witkowska E, Chung N, Schiller P, Izdebski J (2013) Antinociceptive effects of two deltorphins analogs in the tail-immersion test in rats. Peptides 39:103–110
Célérier E, Yazdi MT, Castañé A, Ghozland S, Nyberg F, Maldonado R (2003) Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. Eur J Pharmacol 465:69–81
Listos J, Łupina M, Talarek S, Mazur A, Orzelska-Górka J, Kotlińska J (2019) The mechanisms involved in morphine addiction: an overview. Int J Mol Sci 20:4302
Wood JD, Galligan J (2004) Function of opioids in the enteric nervous system. Neurogastroenterol Motil 16:17–28
James A, Williams J (2020) Basic opioid pharmacology—an update. Br J Pain 14:115–121
Zhang J-l, Wang H, Chen C, Pi H-f, Raun H-l, Zhang P, Wu J-z (2009) Addictive evaluation of cholic acid-verticinone ester, a potential cough therapeutic agent with agonist action of opioid receptor. Acta Pharmacol Sin 30:559–566
Cox BM, Christie MJ, Devi L, Toll L, Traynor JR (2015) Challenges for opioid receptor nomenclature: IUPHAR review 9. Br J Pharmacol 172:317–323
Dhaliwal A, Gupta M (2019) Physiology, opioid receptor. StatPearls Publishing, Treasure Island
Raehal KM, Bohn LM (2011) The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60:58–65
Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL (2011) The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590
DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen X-T, Pitis PM, Gotchev D, Yuan C (2013) AG protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717
Violin JD, Crombie AL, Soergel DG, Lark MW (2014) Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci 35:308–316
Khroyan TV, Wu J, Polgar WE, Cami-Kobeci G, Fotaki N, Husbands SM, Toll L (2015) BU 08073 a buprenorphine analogue with partial agonist activity at μ-receptors in vitro but long-lasting opioid antagonist activity in vivo in mice. Br J Pharmacol 172:668–680
Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190
Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch JA, Sames D (2016) Synthetic and receptor signaling explorations of the mitragyna alkaloids: mitragynine as an atypical molecular framework for opioid receptor modulators. J Am Chem Soc 138:6754–6764
Berger AC, Whistler JL (2010) How to design an opioid drug that causes reduced tolerance and dependence. Ann Neurol 67:559–569
Stafford K, Gomes AB, Shen J, Yoburn BC (2001) μ-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav 69:233–237
He L, Fong J, von Zastrow M, Whistler JL (2002) Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell 108:271–282
Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ (2013) Regulation of µ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 65:223–254
Allouche S, Noble F, Marie N (2014) Opioid receptor desensitization: mechanisms and its link to tolerance. Front Pharmacol 5:280
Bohn LM, Gainetdinov RR, Lin F-T, Lefkowitz RJ, Caron MG (2000) m-Opioid receptor desensitization by b-arrestin-2 determines morphine tolerance but not dependence. Nature. https://doi.org/10.1038/35047086
Al-Hasani R, Bruchas MR (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. J Am Soc Anesthesiol 115:1363–1381
Nabemoto M, Mashimo M, Someya A, Nakamura H, Hirabayashi T, Fujino H, Kaneko M, Okuma Y, Saito T, Yamaguchi N (2008) Release of arachidonic acid by 2-arachidonoyl glycerol and HU210 in PC12 cells; roles of Src, phospholipase C and cytosolic phospholipase A2α. Eur J Pharmacol 590:1–11
Ghosh N, Kesh K, Singh PK, Sharma U, Chupikova I, Ramakrishnan S, Roy S (2022) Morphine use induces gastric microbial dysbiosis driving gastric inflammation through TLR2 signaling which is attenuated by proton pump inhibitor. Br J Pharmacol. https://doi.org/10.1111/bph.16025
Anselmi L, Jaramillo I, Palacios M, Huynh J, Sternini C (2013) Ligand-induced μ opioid receptor internalization in enteric neurons following chronic treatment with the opiate fentanyl. J Neurosci Res 91:854–860
Zuo ZJA (2005) The role of opioid receptor internalization and β-arrestins in the development of opioid tolerance. Anesth Analg 101:728–734
Fan X, Zhang J, Zhang X, Yue W, Ma LJN (2003) Differential regulation of β-arrestin 1 and β-arrestin 2 gene expression in rat brain by morphine. Neuroscience 117:383–389
Bulka A, Plesan A, Xu X-J, Wiesenfeld-Hallin ZJP (2002) Reduced tolerance to the anti-hyperalgesic effect of methadone in comparison to morphine in a rat model of mononeuropathy. Pain 95:103–109
Uchtenhagen A (2009) Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva: World Health Organization
Bono A, Cuffari SJD (1997) Effectiveness and tolerance of tramadol in cancer pain. A comparative study with respect to buprenorphine. Drugs 53:40–49
Garry P, Ezra M, Rowland M, Westbrook J, Pattinson K (2015) The role of the nitric oxide pathway in brain injury and its treatment—from bench to bedside. Exp Neurol 263:235–243
Skrabalova J, Drastichova Z, Novotny J (2013) Morphine as a potential oxidative stress-causing agent. Mini-Rev Org Chem 10:367–372
Goudas LC, Langlade A, Serrie A, Matson W, Milbury P, Thurel C, Sandouk P, Carr DB (1999) Acute decreases in cerebrospinal fluid glutathione levels after intracerebroventricular morphine for cancer pain. Anesth Analg 89:1209–1215
Abdel-Zaher AO, Mostafa MG, Farghaly HS, Hamdy MM, Abdel-Hady RH (2013) Role of oxidative stress and inducible nitric oxide synthase in morphine-induced tolerance and dependence in mice. Effect of alpha-lipoic acid. Behav Brain Res 247:17–26
Zeng X-S, Geng W-S, Wang Z-Q, Jia J-J (2020) Morphine addiction and oxidative stress: the potential effects of thioredoxin-1. Front Pharmacol 11:82
Džoljić E, Grabatinić I, Kostić V (2015) Why is nitric oxide important for our brain? Funct Neurol 30:159
Dambisya YM, Lee TL (1996) Role of nitric oxide in the induction and expression of morphine tolerance and dependence in mice. Br J Pharmacol 117:914–918
Hosseinzadeh H, Imenshahidi M, Hosseini M, Razavi BM (2012) Effect of linalool on morphine tolerance and dependence in mice. Phytother Res 26:1399–1404
Cappendijk SL, de Vries R, Dzoljic MR (1993) Inhibitory effect of nitric oxide (NO) synthase inhibitors on naloxone-precipitated withdrawal syndrome in morphine-dependent mice. Neurosci Lett 162:97–100
Chen C, Fan Q, Nong Z, Chen W, Li Y, Huang L, Feng D, Pan X, Lan S (2018) Hyperbaric oxygen attenuates withdrawal symptoms by regulating monoaminergic neurotransmitters and NO signaling pathway at nucleus accumbens in morphine-dependent rats. Neurochem Res 43:531–539
Ozdemir E, Bagcivan I, Durmus N, Altun A, Gursoy S (2011) The nitric oxide–cGMP signaling pathway plays a significant role in tolerance to the analgesic effect of morphine. Can J Physiol Pharmacol 89:89–95
Liang D-Y, Clark JD (2004) Modulation of the NO/CO-cGMP signaling cascade during chronic morphine exposure in mice. Neurosci Lett 365:73–77
Zhou Q, Sheng M (2013) NMDA receptors in nervous system diseases. Neuropharmacology 74:69–75
Popik P, Skolnick P (1996) The NMDA antagonist memantine blocks the expression and maintenance of morphine dependence. Pharmacol Biochem Behav 53:791–797
Esmaili-Shahzade-Ali-Akbari P, Hosseinzadeh H, Mehri S (2021) Effect of suvorexant on morphine tolerance and dependence in mice: role of NMDA, AMPA, ERK and CREB proteins. Neurotoxicology 84:64–72
Trujillo KA, Akil H (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251:85–87
Heneka MT, Klockgether T, Feinstein DL (2000) Peroxisome proliferator-activated receptor-γ ligands reduce neuronal inducible nitric oxide synthase expression and cell deathin vivo. J Neurosci 20:6862–6867
Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW (2009) Expression of peroxisome proliferator-activated receptor-γ in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 150:707–712
Janani C, Kumari BR (2015) PPAR gamma gene–a review. Diabetes Metab Syndr 9:46–50
Ray LA, Roche DJ, Heinzerling K, Shoptaw S (2014) Opportunities for the development of neuroimmune therapies in addiction. Int Rev Neurobiol 118:381–401
Willson TM, Brown PJ, Sternbach DD, Henke BR (2000) The PPARs: from orphan receptors to drug discovery. J Med Chem 43:527–550
Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G (2011) Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiat 69:642–649
Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S (2007) Peroxisome proliferator-activated receptor gamma activation relieves expression of behavioral sensitization to methamphetamine in mice. Neuropsychopharmacology 32:1133–1140
Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ (2012) Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology 37:1838–1847
Ghavimi H, Hassanzadeh K, Maleki-Dizaji N, Azarfardian A, Ghasami S, Zolali E, Charkhpour M (2014) Pioglitazone prevents morphine antinociception tolerance and withdrawal symptoms in rats. Naunyn Schmiedebergs Arch Pharmacol 387:811–821
Liu C-H, Cherng C-H, Lin S-L, Yeh C-C, Wu C-T, Tai Y-H, Wong C-S (2011) N-methyl-D-aspartate receptor antagonist MK-801 suppresses glial pro-inflammatory cytokine expression in morphine-tolerant rats. Pharmacol Biochem Behav 99:371–380
Watkins LR, Hutchinson MR, Rice KC, Maier SF (2009) The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 30:581–591
Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S (2020) Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol 11:1455
Charkhpour M, Ghavimi H, Ghanbarzadeh S, Yousefi B, Khorrami A, Mesgari M, Hassanzadeh K (2015) Protective effect of pioglitazone on morphine-induced neuroinflammation in the rat lumbar spinal cord. J Biomed Sci 22:1–6
Ghavimi H, Charkhpour M, Ghasemi S, Mesgari M, Hamishehkar H, Hassanzadeh K, Arami S, Hassanzadeh K (2015) Pioglitazone prevents morphine antinociceptive tolerance via ameliorating neuroinflammation in rat cerebral cortex. Pharmacol Rep 67:78–84
Verster JC, Scholey A, Dahl TA, Iversen JM (2021) Functional observation after morphine withdrawal: effects of SJP-005. Psychopharmacology 238:1449–1460
de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G, Ciccocioppo R (2017) Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology 234:223–234
Prickaerts J, Van Staveren W, Şik A, Markerink-van Ittersum M, Niewöhner U, Van der Staay F, Blokland A, De Vente J (2002) Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 113:351–361
Rehni AK, Bhateja P, Singh TG, Singh N (2008) Nuclear factor-κ-B inhibitor modulates the development of opioid dependence in a mouse model of naloxone-induced opioid withdrawal syndrome. Behav Pharmacol 19:265–269
Sawaya BE, Deshmane SL, Mukerjee R, Fan S, Khalili K (2009) TNF alpha production in morphine-treated human neural cells is NF-κB-dependent. J Neuroimmune Pharmacol 4:140–149
Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive–sensitization view. Addiction 95:91–117
Sahraei H, Zarei F, Eidi A, Oryan S, Shams J, Khoshbaten A, Zarrindast M-R (2007) The role of nitric oxide within the nucleus accumbens on the acquisition and expression of morphine-induced place preference in morphine sensitized rats. Eur J Pharmacol 556:99–106
Huston JP, de Souza Silva MA, Topic B, Müller CP (2013) What’s conditioned in conditioned place preference? Trends Pharmacol Sci 34:162–166
Robinson TE, Berridge KC (2008) The incentive sensitization theory of addiction: some current issues. Phil Trans R Soc B 363:3137–3146
Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151:99–120
Eisch AJ, Harburg GC (2006) Opiates, psychostimulants, and adult hippocampal neurogenesis: insights for addiction and stem cell biology. Hippocampus 16:271–286
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449
Reisi Z, Bani-Ardalan M, Zarepour L, Haghparast A (2014) Involvement of D1/D2 dopamine receptors within the nucleus accumbens and ventral tegmental area in the development of sensitization to antinociceptive effect of morphine. Pharmacol Biochem Behav 118:16–21
Borgkvist A, Valjent E, Santini E, Hervé D, Girault J-A, Fisone G (2008) Delayed, context-and dopamine D1 receptor-dependent activation of ERK in morphine-sensitized mice. Neuropharmacology 55:230–237
Jeziorski M, White FJ, Wolf ME (1994) MK-801 prevents the development of behavioral sensitization during repeated morphine administration. Synapse 16:137–147
Carlezon WA Jr, Rasmussen K, Nestler EJ (1999) AMPA antagonist LY293558 blocks the development, without blocking the expression, of behavioral sensitization to morphine. Synapse 31:256–262
Mattick RP, Breen C, Kimber J, Davoli M (2009) Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002209.pub2
Pilarinos A, Kwa Y, Joe R, Dong H, Grant C, Fast D, Buxton JA, DeBeck K (2023) Methadone maintenance treatment discontinuation among young people who use opioids in Vancouver, Canada. Can J Psychiatry 68:89–100
Faccio E, Aquili L, Rocelli M (2022) When the non-sharing of therapeutic goals becomes the problem: the story of a consumer and his addiction to methadone. J Psychiatr Ment Health Nurs 29:174–180
Kreutzwiser D, Tawfic QA (2020) Methadone for pain management: a pharmacotherapeutic review. CNS Drugs 34:827–839
Eap CB, Buclin T, Baumann P (2002) Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet 41:1153–1193
Srivastava AB, Mariani JJ, Levin FR (2020) New directions in the treatment of opioid withdrawal. Lancet 395:1938–1948
Gowing LR, Ali RL, White JM (2000) The management of opioid withdrawal. Drug Alcohol Rev 19:309–318
Grim TW, Schmid CL, Stahl EL, Pantouli F, Ho J-H, Acevedo-Canabal A, Kennedy NM, Cameron MD, Bannister TD, Bohn LM (2020) AG protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuropsychopharmacology 45:416–425
Kim SU, De Vellis J (2009) Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res 87:2183–2200
Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K (2019) Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. https://doi.org/10.1155/2019/9628536
Wei X, Yang X, Han Z-p, Qu F-f, Shao L, Shi Y-f (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 34:747–754
Saeedi P, Halabian R, Fooladi AAI (2019) A revealing review of mesenchymal stem cells therapy, clinical perspectives and modification strategies. Stem Cell Investig 6:34
Li F, Liu L, Cheng K, Chen Z, Cheng J (2018) The use of stem cell therapy to reverse opioid tolerance. Clin Pharmacol Ther 103:971–974
Hua Z, Liu L, Shen J, Cheng K, Liu A, Yang J, Wang L, Qu T, Yang H, Li Y (2016) Mesenchymal stem cells reversed morphine tolerance and opioid-induced hyperalgesia. Sci Rep 6:1–13
Yang H, Sun J, Chen H, Wang F, Li Y, Wang H, Qu T (2019) Mesenchymal stem cells from bone marrow attenuated the chronic morphine-induced cAMP accumulation in vitro. Neurosci Lett 698:76–80
Herberts CA, Kwa MS, Hermsen HP (2011) Risk factors in the development of stem cell therapy. J Transl Med 9:1–14
Kamarajan C, Porjesz B (2015) Advances in electrophysiological research. Alcohol Res 37:53
Stewart JL, May AC (2016) Electrophysiology for addiction medicine: from methodology to conceptualization of reward deficits. Prog Brain Res 224:67–84
Suk H-J, Boyden ES, van Welie I (2019) Advances in the automation of whole-cell patch clamp technology. J Neurosci Methods 326:108357
Gross J (2014) Analytical methods and experimental approaches for electrophysiological studies of brain oscillations. J Neurosci Methods 228:57–66
Kalivas PW (2004) Recent understanding in the mechanisms of addiction. Curr Psychiatry Rep 6:347–351
Lane D, Tortorici V, Morgan M (2004) Behavioral and electrophysiological evidence for tolerance to continuous morphine administration into the ventrolateral periaqueductal gray. Neuroscience 125:63–69
Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F (2011) Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci 108:16446–16450
Georges F, Le Moine C, Aston-Jones G (2006) No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci 26:5720–5726
Li Z, Luan W, Chen Y, Chen M, Dong Y, Lai B, Ma L, Zheng P (2011) Chronic morphine treatment switches the effect of dopamine on excitatory synaptic transmission from inhibition to excitation in pyramidal cells of the basolateral amygdala. J Neurosci 31:17527–17536
Zhao Y, Inayat S, Dikin D, Singer J, Ruoff R, Troy J (2008) Patch clamp technique: review of the current state of the art and potential contributions from nanoengineering. Proc Inst Mech Eng Part N 222:1–11
Kodandaramaiah SB, Franzesi GT, Chow BY, Boyden ES, Forest CR (2012) Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat Methods 9:585–587
Kaski SW, White AN, Gross JD, Siderovski DP (2021) Potential for Kappa opioid receptor agonists to engineer non-addictive analgesics: a narrative review. Anesth Analg 132:406
Witkowska E, Godlewska M, Osiejuk J, Gątarz S, Wileńska B, Kosińska K, Starnowska-Sokół J, Piotrowska A, Lipiński PF, Matalińska J (2022) Bifunctional opioid/melanocortin peptidomimetics for use in neuropathic pain: variation in the type and length of the linker connecting the two pharmacophores. Int J Mol Sci 23:674
Smith MT, Kong D, Kuo A, Imam MZ, Williams CM (2023) Multitargeted opioid ligand discovery as a strategy to retain analgesia and reduce opioid-related adverse effects. J Med Chem 66:3746–3784
Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L (2009) Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/μ-opioid receptor agonists. J Pharmacol Exp Ther 331:946–953
Varga BR, Streicher JM, Majumdar S (2023) Strategies towards safer opioid analgesics—a review of old and upcoming targets. Br J Pharmacol 180:975–993
Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, Czoty PW, Kishioka S, Zaveri NT, Ko M-C (2018) A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med 10:e3483
Kruegel AC, Grundmann O (2018) The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 134:108–120
Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, Ansonoff M, Gassaway MM, Pintar JE, Pasternak GW (2019) 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent Sci 5:992–1001
Basheer M, Hassan Z, Gam L-H (2023) Upregulation of brain’s calcium binding proteins in mitragynine dependence: a potential cellular mechanism to addiction. Int J Med Sci 20:102–113
Hassan Z, Muzaimi M, Navaratnam V, Yusoff NH, Suhaimi FW, Vadivelu R, Vicknasingam BK, Amato D, von Hörsten S, Ismail NI (2013) From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev 37:138–151
Swogger MT, Hart E, Erowid F, Erowid E, Trabold N, Yee K, Parkhurst KA, Priddy BM, Walsh Z (2015) Experiences of kratom users: a qualitative analysis. J Psychoact Drugs 47:360–367
Stefanucci A, Minosi P, Pieretti S, Tanguturi P, Molnar G, Scioli G, Marinaccio L, Della Valle A, Streicher JM, Mollica A (2023) Design of analgesic trivalent peptides with low withdrawal symptoms: probing the antinociceptive profile of novel linear and cyclic peptides as opioid pan ligands. ACS Chem Neurosci. https://doi.org/10.1021/acschemneuro.3c00005
de Guglielmo G, Kallupi M, Scuppa G, Stopponi S, Demopulos G, Gaitanaris G, Ciccocioppo R (2014) Analgesic tolerance to morphine is regulated by PPAR γ. Br J Pharmacol 171:5407–5416
Hao X-Q, Wang Z-Y, Chen J-M, Wu N, Li J (2021) Involvement of the nociceptin opioid peptide receptor in morphine-induced antinociception, tolerance and physical dependence in female mice. Metab Brain Dis 36:2243–2253
Hassan R, Pike See C, Sreenivasan S, Mansor SM, Müller CP, Hassan Z (2020) Mitragynine attenuates morphine withdrawal effects in rats—a comparison with methadone and buprenorphine. Front Psych 11:411
Gabra BH, Afify EA, Daabees TT, Abou Zeit-Har MS (2005) The role of the NO/NMDA pathways in the development of morphine withdrawal induced by naloxone in vitro. Pharmacol Res 51:319–327
Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, Hernandez-Viadel M, Erceg S, Sánchez-Perez AM, Felipo V (2003) Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int 43:493–499
Song K, Li Y, Zhang H, An N, Wei Y, Wang L, Tian C, Yuan M, Sun Y, Xing Y (2020) Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxid Med Cell Longev. https://doi.org/10.1155/2020/4356386
Acknowledgements
BioRender software (Canada) was used for the construction of graphical portion of the manuscript.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
IB and MA participated in drafting the manuscript. BM and MIK conceptualized the study and were involved in the editing, proof reading and final preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
All authors have declared no conflict or competing financial interest whatsoever, which can negatively influence the current work reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badshah, I., Anwar, M., Murtaza, B. et al. Molecular mechanisms of morphine tolerance and dependence; novel insights and future perspectives. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04810-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04810-3