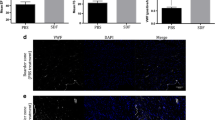
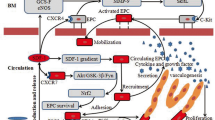
Abstract
Cytokine release syndrome (CRS) is an acute systemic inflammatory reaction in which hyperactivated immune cells suddenly release a large amount of cytokines, leading to exaggerated inflammatory responses, multiple organ dysfunction, and even death. Although palliative treatment strategies have significantly reduced the overall mortality, novel targeted treatment regimens with superior therapy efficacy are urgently needed. Vascular endothelial cells (ECs) are important target cells of systemic inflammation, and their destruction is considered to be the initiating event underlying many serious complications of CRS. Mesenchymal stem/stromal cells (MSCs) are multipotent cells with self-renewing differentiation capacity and immunomodulatory properties. MSC transplantation can effectively suppress the activation of immune cells, reduce the bulk release of cytokines, and repair damaged tissues and organs. Here, we review the molecular mechanisms underlying CRS-induced vascular endothelial injury and discuss potential treatments using MSCs. Preclinical studies demonstrate that MSC therapy can effectively repair endothelium damage and thus reduce the incidence and severity of ensuing CRS-induced complications. This review highlights the therapeutic role of MSCs in fighting against CRS-induced EC damage, and summarizes the possible therapeutic formulations of MSCs for improved efficacy in future clinical trials.






Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- CAR-T:
-
Chimeric antigen receptor T-cell
- CCN1:
-
CCN family member 1
- CD33:
-
Cluster of differentiation 33
- COVID-19:
-
The coronavirus disease 2019
- CRS:
-
Cytokine release syndrome
- CXC:
-
C-X-C motif chemokine
- CXCR:
-
CXC receptor
- CYR61:
-
Cysteine-rich angiogenic inducer 61
- EC:
-
Endothelial cells
- HGF:
-
Hepatocyte growth factor
- HLA-DR:
-
Human leukocyte antigen—DR isotype
- HLH:
-
Hemophagocytic lymphohistiocytosis
- HO-1:
-
Heme oxidase-1
- HUVEC:
-
Human umbilical vascular endothelial cell
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFN-γ:
-
Interferon-γ
- IL-2:
-
Interleukin-2
- IL-6R:
-
IL-6 receptor
- MAPK:
-
Mitogen-activated protein kinase
- MAS:
-
Macrophage activation syndrome
- MCP-1:
-
Monocyte chemoattractant protein-1
- MIP-1α:
-
Macrophage inflammatory protein-1α
- MRTF-A:
-
Myocardin-related transcription factor-A
- MSC:
-
Mesenchymal stem cell
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor κB
- NK:
-
Natural killer
- NQO1:
-
NADPH-dependent quinone oxidoreductase
- PI3K/Akt:
-
Phosphoinositide-3-kinase–protein kinase B/Akt
- ROCK:
-
Rho-associated kinases
- ROS:
-
Reactive oxygen species
- SDF:
-
Stromal cell-derived factor
- TLS:
-
Tumor lysis syndrome
- TNF-α:
-
Tumor necrosis factor-α
- TSG-6:
-
TNF-stimulated gene 6
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Mansouri V, Yazdanpanah N, Rezaei N (2021) The immunologic aspects of cytokine release syndrome and graft versus host disease following car T cell therapy. Int Rev Immunol. https://doi.org/10.1080/08830185.2021.1984449
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration UK (2020) Covid-19: consider cytokine storm syndromes and immunosuppression. Lancet 395:1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL (2014) Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188–195. https://doi.org/10.1182/blood-2014-05-552729
Ding DC, Shyu WC, Lin SZ (2011) Mesenchymal stem cells. Cell Transplant 20:5–14. https://doi.org/10.3727/096368910x
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy 8:315–317. https://doi.org/10.1080/14653240600855905
Mushahary D, Spittler A, Kasper C, Weber V, Charwat V (2018) Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 93:19–31. https://doi.org/10.1002/cyto.a.23242
Alvandi Z, Bischoff J (2021) Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler Thromb Vasc Biol 41:2357–2369. https://doi.org/10.1161/ATVBAHA.121.313788
Piera-Velazquez S, Jimenez SA (2019) Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev 99:1281–1324. https://doi.org/10.1152/physrev.00021.2018
Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI (2019) Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 4:22. https://doi.org/10.1038/s41536-019-0083-6
Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V (2020) Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol 8:43. https://doi.org/10.3389/fbioe.2020.00043
Rodriguez-Fuentes DE, Fernandez-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldana HA (2021) Mesenchymal stem cells current clinical applications: a systematic review. Arch Med Res 52:93–101. https://doi.org/10.1016/j.arcmed.2020.08.006
Song N, Wakimoto H, Rossignoli F, Bhere D, Ciccocioppo R, Chen KS, Khalsa JK, Mastrolia I, Samarelli AV, Dominici M, Shah K (2021) Mesenchymal stem cell immunomodulation. in pursuit of controlling covid-19 related cytokine storm. Stem Cells 39:707–722. https://doi.org/10.1002/stem.3354
England JT, Abdulla A, Biggs CM, Lee AYY, Hay KA, Hoiland RL, Wellington CL, Sekhon M, Jamal S, Shojania K, Chen LYC (2021) Weathering the covid-19 storm: lessons from hematologic cytokine syndromes. Blood Rev 45:100707. https://doi.org/10.1016/j.blre.2020.100707
Tang L, Yin Z, Hu Y, Mei H (2020) Controlling cytokine storm is vital in covid-19. Front Immunol 11:570993. https://doi.org/10.3389/fimmu.2020.570993
Shimabukuro-Vornhagen A, Godel P, Subklewe M, Stemmler HJ, Schlosser HA, Schlaak M, Kochanek M, Boll B, von Bergwelt-Baildon MS (2018) Cytokine release syndrome. J Immunother Cancer 6:56. https://doi.org/10.1186/s40425-018-0343-9
Karki R, Kanneganti TD (2021) The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunol 42:681–705. https://doi.org/10.1016/j.it.2021.06.001
Thepmankorn P, Bach J, Lasfar A, Zhao X, Souayah S, Chong ZZ, Souayah N (2021) Cytokine storm induced by sars-cov-2 infection: the spectrum of its neurological manifestations. Cytokine 138:155404. https://doi.org/10.1016/j.cyto.2020.155404
Kunchok A, Krecke KN, Flanagan EP, Jitprapaikulsan J, Lopez-Chiriboga AS, Chen JJ, Weinshenker BG, Pittock SJ (2020) Does area postrema syndrome occur in myelin oligodendrocyte glycoprotein-igg-associated disorders (Mogad)? Neurology 94:85–88. https://doi.org/10.1212/wnl.0000000000008786
Fajgenbaum DC, June CH (2020) Cytokine storm. N Engl J Med 383:2255–2273. https://doi.org/10.1056/NEJMra2026131
Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, Ye Z, Qian Q (2019) Current progress in Car-T cell therapy for solid tumors. Int J Biol Sci 15:2548–2560. https://doi.org/10.7150/ijbs.34213
Wang X, Riviere I (2016) Clinical manufacturing of car T cells: foundation of a promising therapy. Mol Ther Oncolytics 3:16015. https://doi.org/10.1038/mto.2016.15
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P, Flinn IW, Farooq U, Goy A, McSweeney PA, Munoz J, Siddiqi T, Chavez JC, Herrera AF, Bartlett NL, Wiezorek JS, Navale L, Xue A, Jiang Y, Bot A, Rossi JM, Kim JJ, Go WY, Neelapu SS (2019) Long-Term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (Zuma-1): a single-arm, multicentre, phase 1–2 Trial. Lancet Oncol 20:31–42. https://doi.org/10.1016/S1470-2045(18)30864-7
Ying Z, Yang H, Guo Y, Li W, Zou D, Zhou D, Wang Z, Zhang M, Wu J, Liu H, Zhang P, Yang S, Zhou Z, Zheng H, Song Y, Zhu J (2021) Relmacabtagene Autoleucel (Relma-Cel) Cd19 Car-T therapy for adults with heavily pretreated relapsed/refractory large b-cell lymphoma in China. Cancer Med 10:999–1011. https://doi.org/10.1002/cam4.3686
Siegler EL, Kenderian SS (2020) Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T Cell therapy: insights into mechanisms and novel therapies. Front Immunol 11:1973. https://doi.org/10.3389/fimmu.2020.01973
Wang J, Doran J (2021) The many faces of cytokine release syndrome-related coagulopathy. Clin Hematol Int 3:3–12. https://doi.org/10.2991/chi.k.210117.001
Kang S, Kishimoto T (2021) Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med 53:1116–1123. https://doi.org/10.1038/s12276-021-00649-0
Lo CW, Chen MW, Hsiao M, Wang S, Chen CA, Hsiao SM, Chang JS, Lai TC, Rose-John S, Kuo ML, Wei LH (2011) Il-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res 71:424–434. https://doi.org/10.1158/0008-5472.CAN-10-1496
Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA (2003) Protective effects of Il-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol 170:503–507. https://doi.org/10.4049/jimmunol.170.1.503
Kang S, Tanaka T, Inoue H, Ono C, Hashimoto S, Kioi Y, Matsumoto H, Matsuura H, Matsubara T, Shimizu K, Ogura H, Matsuura Y, Kishimoto T (2020) Il-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A 117:22351–22356. https://doi.org/10.1073/pnas.2010229117
Paleolog EM, Crossman DC, McVey JH, Pearson JD (1990) Differential regulation by cytokines of constitutive and stimulated secretion of von willebrand factor from endothelial cells. Blood 75:688–695
Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF (2004) Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von willebrand factor multimers under flow. Blood 104:100–106. https://doi.org/10.1182/blood-2004-01-0107
Norooznezhad AH, Mansouri K (2021) Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (covid-19). Microvasc Res 137:104188. https://doi.org/10.1016/j.mvr.2021.104188
Teuwen LA, Geldhof V, Pasut A, Carmeliet P (2020) Covid-19: the vasculature unleashed. Nat Rev Immunol 20:389–391. https://doi.org/10.1038/s41577-020-0343-0
Ruhl L, Pink I, Kuhne JF, Beushausen K, Keil J, Christoph S, Sauer A, Boblitz L, Schmidt J, David S, Jack HM, Roth E, Cornberg M, Schulz TF, Welte T, Hoper MM, Falk CS (2021) Endothelial dysfunction contributes to severe covid-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct Target Ther 6:418. https://doi.org/10.1038/s41392-021-00819-6
Biedermann BC (2008) Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol 21:129–138. https://doi.org/10.1016/j.beha.2008.02.003
Hong F, Shi M, Cao J, Wang Y, Gong Y, Gao H, Li Z, Zheng J, Zeng L, He A, Xu K (2021) Predictive role of endothelial cell activation in cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukaemia. J Cell Mol Med 25:11063–11074. https://doi.org/10.1111/jcmm.17029
Cobb DA, Lee DW (2021) Cytokine release syndrome biology and management. Cancer J 27:119–125. https://doi.org/10.1097/PPO.0000000000000515
Riegler LL, Jones GP, Lee DW (2019) Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag 15:323–335. https://doi.org/10.2147/TCRM.S150524
He Y, Lin S, Ao Q, He X (2020) The co-culture of Ascs and Epcs promotes vascularized bone regeneration in critical-sized bone defects of cranial bone in rats. Stem Cell Res Ther 11:338. https://doi.org/10.1186/s13287-020-01858-6
Wang Z, Han L, Sun T, Wang W, Li X, Wu B (2021) Osteogenic and angiogenic lineage differentiated adipose-derived stem cells for bone regeneration of calvarial defects in rabbits. J Biomed Mater Res A 109:538–550. https://doi.org/10.1002/jbm.a.37036
Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (2020) Cytokine release syndrome in severe covid-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 55:105954. https://doi.org/10.1016/j.ijantimicag.2020.105954
Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, Farrell AT, Pazdur R (2018) FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23:943–947. https://doi.org/10.1634/theoncologist.2018-0028
Pelaia C, Calabrese C, Garofalo E, Bruni A, Vatrella A, Pelaia G (2021) Therapeutic role of tocilizumab in Sars-Cov-2-induced cytokine storm: rationale and current evidence. Int J Mol Sci. https://doi.org/10.3390/ijms22063059
Du P, Geng J, Wang F, Chen X, Huang Z, Wang Y (2021) Role of Il-6 inhibitor in treatment of covid-19-related cytokine release syndrome. Int J Med Sci 18:1356–1362. https://doi.org/10.7150/ijms.53564
Zhou Z, Price CC (2020) Overview on the use of Il-6 agents in the treatment of patients with cytokine release syndrome (Crs) and pneumonitis related to covid-19 disease. Expert Opin Investig Drugs 29:1407–1412. https://doi.org/10.1080/13543784.2020.1840549
Teachey DT, Bishop MR, Maloney DG, Grupp SA (2018) Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘all.’ Nat Rev Clin Oncol 15:218. https://doi.org/10.1038/nrclinonc.2018.19
Zhang L, Wang S, Xu J, Zhang R, Zhu H, Wu Y, Zhu L, Li J, Chen L (2021) Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy. Exp Hematol Oncol 10:16. https://doi.org/10.1186/s40164-021-00209-2
Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, Wang LL, Han WD (2015) Treatment of Cd33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol Ther 23:184–191. https://doi.org/10.1038/mt.2014.164
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Locke FL, Lin Y, Jain N, Daver N, Gulbis AM, Adkins S, Rezvani K, Hwu P, Shpall EJ (2018) Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘all.’ Nat Rev Clin Oncol 15:218. https://doi.org/10.1038/nrclinonc.2018.20
Frey N (2017) cytokine release syndrome: who is at risk and how to treat. Best Pract Res Clin Haematol 30:336–340. https://doi.org/10.1016/j.beha.2017.09.002
Liu S, Deng B, Yin Z, Pan J, Lin Y, Ling Z, Wu T, Chen D, Chang AH, Gao Z, Song Y, Zhao Y, Tong C (2020) Corticosteroids do not influence the efficacy and kinetics of car-T cells for b-cell acute lymphoblastic leukemia. Blood Cancer J 10:15. https://doi.org/10.1038/s41408-020-0280-y
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, Traversari C, Bordignon C, Ciceri F, Ostuni R, Bonini C, Casucci M, Bondanza A (2018) Monocyte-derived Il-1 and Il-6 are differentially required for cytokine-release syndrome and neurotoxicity due to car T cells. Nat Med 24:739–748. https://doi.org/10.1038/s41591-018-0036-4
Xiao X, He X, Li Q, Zhang H, Meng J, Jiang Y, Deng Q, Zhao M (2019) Plasma exchange can be an alternative therapeutic modality for severe cytokine release syndrome after chimeric antigen receptor-T cell infusion: a case report. Clin Cancer Res 25:29–34. https://doi.org/10.1158/1078-0432.ccr-18-1379
Liu Y, Chen X, Wang D, Li H, Huang J, Zhang Z, Qiao Y, Zhang H, Zeng Y, Tang C, Yang S, Wan X, Chen YH, Zhang Y (2018) Hemofiltration successfully eliminates severe cytokine release syndrome following Cd19 Car-T-cell therapy. J Immunother 41:406–410. https://doi.org/10.1097/cji.0000000000000243
Xu R, Feng Z, Wang FS (2022) Mesenchymal stem cell treatment for covid-19. EBioMedicine 77:103920. https://doi.org/10.1016/j.ebiom.2022.103920
Liu Q, Ma F, Zhong Y, Wang G, Hu L, Zhang Y, Xie J (2023) Efficacy and safety of human umbilical cord-derived mesenchymal stem cells for covid-19 pneumonia: a meta-analysis of randomized controlled trials. Stem Cell Res Ther 14:118. https://doi.org/10.1186/s13287-023-03286-8
Zhang Z, Shao S, Liu X, Tong Z (2023) Effect and safety of mesenchymal stem cells for patients with covid-19: systematic review and meta-analysis with trial sequential analysis. J Med Virol. https://doi.org/10.1002/jmv.28702
Li TT, Zhang B, Fang H, Shi M, Yao WQ, Li Y, Zhang C, Song J, Huang L, Xu Z, Yuan X, Fu JL, Zhen C, Zhang Y, Wang ZR, Zhang ZY, Yuan MQ, Dong T, Bai R, Zhao L, Cai J, Dong J, Zhang J, Xie WF, Li Y, Shi L, Wang FS (2023) Human mesenchymal stem cell therapy in severe covid-19 patients: 2-year follow-up results of a randomized, double-blind placebo-controlled trial. EBioMedicine 92:104600. https://doi.org/10.1016/j.ebiom.2023.104600
Jeyaraman M, John A, Koshy S, Ranjan R, Anudeep TC, Jain R, Swati K, Jha NK, Sharma A, Kesari KK, Prakash A, Nand P, Jha SK, Reddy PH (2021) Fostering mesenchymal stem cell therapy to halt cytokine storm in covid-19. Biochim Biophys Acta Mol Basis Dis 1867:166014. https://doi.org/10.1016/j.bbadis.2020.166014
Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, Mariana N, Antarianto RD, Liem IK, Kispa T, Mujadid F, Novialdi N, Luviah E, Kurniawati T, Lubis AMT, Rahmatika D (2021) Umbilical cord mesenchymal stromal cells as critical covid-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med 10:1279–1287. https://doi.org/10.1002/sctm.21-0046
Hugues FC, Le Jeunne C, Pradalier A, Moulin M, Mauduit B (1986) Drug-induced bronchial spasm and asthma. Therapie 41:241–246
Cheng X, Jiang M, Long L, Meng J (2021) Potential roles of mesenchymal stem cells and their exosomes in the treatment of covid-19. Front Biosci (Landmark Ed) 26:948–961. https://doi.org/10.52586/4999
Arabpour M, Saghazadeh A, Rezaei N (2021) Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol 97:107823. https://doi.org/10.1016/j.intimp.2021.107823
Chu M, Wang H, Bian L, Huang J, Wu D, Zhang R, Fei F, Chen Y, Xia J (2022) Nebulization therapy with umbilical cord mesenchymal stem cell-derived exosomes for covid-19 pneumonia. Stem Cell Rev Rep 18:2152–2163. https://doi.org/10.1007/s12015-022-10398-w
Park JH, Choi Y, Lim CW, Park JM, Yu SH, Kim Y, Han HJ, Kim CH, Song YS, Kim C, Yu SR, Oh EY, Lee SM, Moon J (2021) Potential therapeutic effect of micrornas in extracellular vesicles from mesenchymal stem cells against Sars-Cov-2. Cells. https://doi.org/10.3390/cells10092393
Baranovskii DS, Klabukov ID, Arguchinskaya NV, Yakimova AO, Kisel AA, Yatsenko EM, Ivanov SA, Shegay PV, Kaprin AD (2022) Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig 9:7. https://doi.org/10.21037/sci-2022-025
Liu H, Yang Y, Jiang J, Wang X, Zhang C, Jiang Y, Hong L, Huang H (2019) Coexistence of a huge venous thromboembolism and bleeding tendency in cytokine release syndrome during car-T therapy. Onco Targets Ther 12:8955–8960. https://doi.org/10.2147/OTT.S223697
Hashmi H, Mirza AS, Darwin A, Logothetis C, Garcia F, Kommalapati A, Mhaskar RS, Bachmeier C, Chavez JC, Shah B, Pinilla-Ibarz J, Khimani F, Lazaryan A, Liu H, Davila ML, Locke FL, Nishihori T, Jain MD (2020) Venous thromboembolism associated with Cd19-directed car T-cell therapy in large b-cell lymphoma. Blood Adv 4:4086–4090. https://doi.org/10.1182/bloodadvances.2020002060
Zhou Y, Liu C, He J, Dong L, Zhu H, Zhang B, Feng X, Weng W, Cheng K, Yu M, Wang H (2020) Klf2(+) stemness maintains human mesenchymal stem cells in bone regeneration. Stem Cells 38:395–409. https://doi.org/10.1002/stem.3120
Wang Q, Zhang W, He G, Sha H, Quan Z (2016) Method for in vitro differentiation of bone marrow mesenchymal stem cells into endothelial progenitor cells and vascular endothelial cells. Mol Med Rep 14:5551–5555. https://doi.org/10.3892/mmr.2016.5953
Diomede F, Marconi GD, Fonticoli L, Pizzicanella J, Merciaro I, Bramanti P, Mazzon E, Trubiani O (2020) Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int J Mol Sci 21:3242. https://doi.org/10.3390/ijms21093242
Ikhapoh IA, Pelham CJ, Agrawal DK (2015) Atherogenic cytokines regulate vegf-a-induced differentiation of bone marrow-derived mesenchymal stem cells into endothelial cells. Stem Cells Int 2015:498328. https://doi.org/10.1155/2015/498328
Wang N, Zhang R, Wang SJ, Zhang CL, Mao LB, Zhuang CY, Tang YY, Luo XG, Zhou H, Zhang TC (2013) Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via rho/myocardin-related transcription factor–a signaling pathway. Int J Biochem Cell Biol 45:1447–1456. https://doi.org/10.1016/j.biocel.2013.04.021
Chen YX, Zeng ZC, Sun J, Zeng HY, Huang Y, Zhang ZY (2015) Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J Radiat Res 56:700–708. https://doi.org/10.1093/jrr/rrv026
Wang C, Li Y, Yang M, Zou Y, Liu H, Liang Z, Yin Y, Niu G, Yan Z, Zhang B (2018) Efficient differentiation of bone marrow mesenchymal stem cells into endothelial cells in vitro. Eur J Vasc Endovasc Surg 55:257–265. https://doi.org/10.1016/j.ejvs.2017.10.012
Böhrnsen F, Schliephake H (2016) Supportive angiogenic and osteogenic differentiation of mesenchymal stromal cells and endothelial cells in monolayer and co-cultures. Int J Oral Sci 8:223–230. https://doi.org/10.1038/ijos.2016.39
Santos RA, Asensi KD, de Barros JHO, de Menezes RCS, Cordeiro IR, Neto JMB, Kasai-Brunswick TH, Goldenberg R (2020) Intrinsic angiogenic potential and migration capacity of human mesenchymal stromal cells derived from menstrual blood and bone marrow. Int J Mol Sci 21:9563. https://doi.org/10.3390/ijms21249563
Jiang F, Ma J, Liang Y, Niu Y, Chen N, Shen M (2015) Amniotic mesenchymal stem cells can enhance angiogenic capacity via mmps in vitro and in vivo. Biomed Res Int 2015:324014. https://doi.org/10.1155/2015/324014
Luo R, Li L, Liu X, Yuan Y, Zhu W, Li L, Liu J, Lu Y, Cheng J, Chen Y (2020) Mesenchymal stem cells alleviate palmitic acid-induced endothelial-to-mesenchymal transition by suppressing endoplasmic reticulum stress. Am J Physiol Endocrinol Metab 319:E961-e980. https://doi.org/10.1152/ajpendo.00155.2020
Park S, Jung SC (2021) New sources, differentiation, and therapeutic uses of mesenchymal stem cells. Int J Mol Sci 22:5288. https://doi.org/10.3390/ijms22105288
Varderidou-Minasian S, Lorenowicz MJ (2020) Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics 10:5979–5997. https://doi.org/10.7150/thno.40122
Liu S, Liu F, Zhou Y, Jin B, Sun Q, Guo S (2020) Immunosuppressive property of Mscs mediated by cell surface receptors. Front Immunol 11:1076. https://doi.org/10.3389/fimmu.2020.01076
Xia L, Meng Q, Xi J, Han Q, Cheng J, Shen J, Xia Y, Shi L (2019) The synergistic effect of electroacupuncture and bone mesenchymal stem cell transplantation on repairing thin endometrial injury in rats. Stem Cell Res Ther 10:244. https://doi.org/10.1186/s13287-019-1326-6
Ling L, Hou J, Liu D, Tang D, Zhang Y, Zeng Q, Pan H, Fan L (2022) Important role of the Sdf-1/Cxcr4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (Had-Mscs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (Poi). Stem Cell Res Ther 13:79. https://doi.org/10.1186/s13287-022-02759-6
Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N (2019) Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci 76:3323–3348. https://doi.org/10.1007/s00018-019-03125-1
Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y (2012) Pre-Treatment with Il-1β enhances the efficacy of Msc transplantation in Dss-induced colitis. Cell Mol Immunol 9:473–481. https://doi.org/10.1038/cmi.2012.40
Ziaei R, Ayatollahi M, Yaghobi R, Sahraeian Z, Zarghami N (2014) Involvement of Tnf-Α in differential gene expression pattern of Cxcr4 on human marrow-derived mesenchymal stem cells. Mol Biol Rep 41:1059–1066. https://doi.org/10.1007/s11033-013-2951-2
Sierra-Parraga JM, Merino A, Eijken M, Leuvenink H, Ploeg R, Moller BK, Jespersen B, Baan CC, Hoogduijn MJ (2020) Reparative effect of mesenchymal stromal cells on endothelial cells after hypoxic and inflammatory injury. Stem Cell Res Ther 11:352. https://doi.org/10.1186/s13287-020-01869-3
Ishiuchi N, Nakashima A, Doi S, Yoshida K, Maeda S, Kanai R, Yamada Y, Ike T, Doi T, Kato Y, Masaki T (2020) Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res Ther 11:130. https://doi.org/10.1186/s13287-020-01642-6
Almalki SG, Agrawal DK (2017) Erk Signaling is required for Vegf-a/Vegfr2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells. Stem Cell Res Ther 8:113. https://doi.org/10.1186/s13287-017-0568-4
Hocking AM (2015) The role of chemokines in mesenchymal stem cell homing to wounds. Adv Wound Care (New Rochelle) 4:623–630. https://doi.org/10.1089/wound.2014.0579
Alexeev V, Donahue A, Uitto J, Igoucheva O (2013) Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues. Cytotherapy 15:171–184. https://doi.org/10.1016/j.jcyt.2012.11.006
Joutoku Z, Onodera T, Matsuoka M, Homan K, Momma D, Baba R, Hontani K, Hamasaki M, Matsubara S, Hishimura R, Iwasaki N (2019) Ccl21/Ccr7 axis regulating juvenile cartilage repair can enhance cartilage healing in adults. Sci Rep 9:5165. https://doi.org/10.1038/s41598-019-41621-3
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, Wang Y (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14:493–507. https://doi.org/10.1038/s41581-018-0023-5
Kadle RL, Abdou SA, Villarreal-Ponce AP, Soares MA, Sultan DL, David JA, Massie J, Rifkin WJ, Rabbani P, Ceradini DJ (2018) Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS ONE 13:e0193178. https://doi.org/10.1371/journal.pone.0193178
Zhang S, Fang J, Liu Z, Hou P, Cao L, Zhang Y, Liu R, Li Y, Shang Q, Chen Y, Feng C, Wang G, Melino G, Wang Y, Shao C, Shi Y (2021) Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the Ido-Tsg6 axis. Stem Cell Res Ther 12:50. https://doi.org/10.1186/s13287-020-02118-3
Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF (2015) Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther 6:10. https://doi.org/10.1186/scrt546
Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia D, Fang S, Xu S (2019) Exosomes from Human Umbilical Cord Mesenchymal Stem Cells Enhance Fracture Healing through Hif-1alpha-Mediated Promotion of Angiogenesis in a Rat Model of Stabilized Fracture. Cell Prolif 52:e12570. https://doi.org/10.1111/cpr.12570
Mizuta Y, Akahoshi T, Guo J, Zhang S, Narahara S, Kawano T, Murata M, Tokuda K, Eto M, Hashizume M, Yamaura K (2020) Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the Pi3k/Akt pathway in endothelial cells. Stem Cell Res Ther 11:508. https://doi.org/10.1186/s13287-020-02015-9
Tan YL, Eng SP, Hafez P, Abdul Karim N, Law JX, Ng MH (2022) Mesenchymal stromal cell mitochondrial transfer as a cell rescue strategy in regenerative medicine: a review of evidence in preclinical models. Stem Cells Transl Med 11:814–827. https://doi.org/10.1093/stcltm/szac044
Farfán N, Carril J, Redel M, Zamorano M, Araya M, Monzón E, Alvarado R, Contreras N, Tapia-Bustos A, Quintanilla ME, Ezquer F, Valdés JL, Israel Y, Herrera-Marschitz M, Morales P (2020) Intranasal administration of mesenchymal stem cell secretome reduces hippocampal oxidative stress, neuroinflammation and cell death, improving the behavioral outcome following perinatal asphyxia. Int J Mol Sci 21:7800. https://doi.org/10.3390/ijms21207800
Alshabibi MA, Khatlani T, Abomaray FM, AlAskar AS, Kalionis B, Messaoudi SA, Khanabdali R, Alawad AO, Abumaree MH (2018) Human decidua basalis mesenchymal stem/stromal cells protect endothelial cell functions from oxidative stress induced by hydrogen peroxide and monocytes. Stem Cell Res Ther 9:275. https://doi.org/10.1186/s13287-018-1021-z
Chance TC, Herzig MC, Christy BA, Delavan C, Rathbone CR, Cap AP, Bynum JA (2020) Human mesenchymal stromal cell source and culture conditions influence extracellular vesicle angiogenic and metabolic effects on human endothelial cells in vitro. J Trauma Acute Care Surg 89:S100-s108. https://doi.org/10.1097/ta.0000000000002661
Liang X, Lin F, Ding Y, Zhang Y, Li M, Zhou X, Meng Q, Ma X, Wei L, Fan H, Liu Z (2021) Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Res Ther 12:295. https://doi.org/10.1186/s13287-021-02366-x
Feng Y, Zhu R, Shen J, Wu J, Lu W, Zhang J, Zhang J, Liu K (2019) Human bone marrow mesenchymal stem cells rescue endothelial cells experiencing chemotherapy stress by mitochondrial transfer via tunneling nanotubes. Stem Cells Dev 28:674–682. https://doi.org/10.1089/scd.2018.0248
Zhu W, Yuan Y, Liao G, Li L, Liu J, Chen Y, Zhang J, Cheng J, Lu Y (2018) Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death Dis 9:837. https://doi.org/10.1038/s41419-018-0861-x
Wu KH, Mo XM, Han ZC, Zhou B (2011) Stem cell engraftment and survival in the ischemic heart. Ann Thorac Surg 92:1917–1925. https://doi.org/10.1016/j.athoracsur.2011.07.012
Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH (2019) Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol 98:151041. https://doi.org/10.1016/j.ejcb.2019.04.002
Wang Q, Li X, Wang Q, Xie J, Xie C, Fu X (2019) Heat shock pretreatment improves mesenchymal stem cell viability by heat shock proteins and autophagy to prevent cisplatin-induced granulosa cell apoptosis. Stem Cell Res Ther 10:348. https://doi.org/10.1186/s13287-019-1425-4
Kim W, Lee SK, Kwon YW, Chung SG, Kim S (2019) Pioglitazone-primed mesenchymal stem cells stimulate cell proliferation, collagen synthesis and matrix gene expression in tenocytes. Int J Mol Sci 20:472. https://doi.org/10.3390/ijms20030472
Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L, Yan C, Xie X, Lin Z, Panayi AC, Mi B, Liu G (2021) Exosomes derived from pioglitazone-pretreated mscs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology 19:150. https://doi.org/10.1186/s12951-021-00894-5
Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E (2017) Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther 8:79. https://doi.org/10.1186/s13287-017-0531-4
Golchin A, Shams F, Karami F (2020) Advancing mesenchymal stem cell therapy with Crispr/Cas9 for clinical trial studies. Adv Exp Med Biol 1247:89–100. https://doi.org/10.1007/5584_2019_459
Hu C, Zhao L, Li L (2021) Genetic modification by overexpression of target gene in mesenchymal stromal cell for treating liver diseases. J Mol Med (Berl) 99:179–192. https://doi.org/10.1007/s00109-020-02031-5
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z (2020) Hif-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther 11:373. https://doi.org/10.1186/s13287-020-01881-7
Qu Q, Wang L, Bing W, Bi Y, Zhang C, Jing X, Liu L (2020) Mirna-126–3p carried by human umbilical cord mesenchymal stem cell enhances endothelial function through exosome-mediated mechanisms in vitro and attenuates vein graft neointimal formation in vivo. Stem Cell Res Ther 11:464. https://doi.org/10.1186/s13287-020-01978-z
Kim JY, Choi JH, Kim SH, Park H, Lee D, Kim GJ (2021) Efficacy of gene modification in placenta-derived mesenchymal stem cells based on nonviral electroporation. Int J Stem Cells 14:112–118. https://doi.org/10.15283/ijsc20117
Ryu NE, Lee SH, Park H (2019) Spheroid culture system methods and applications for mesenchymal stem cells. Cells 8:1620. https://doi.org/10.3390/cells8121620
Chan YH, Lee YC, Hung CY, Yang PJ, Lai PC, Feng SW (2021) Three-dimensional spheroid culture enhances multipotent differentiation and stemness capacities of human dental pulp-derived mesenchymal stem cells by modulating Mapk and Nf-Kb signaling pathways. Stem Cell Rev Rep 17:1810–1826. https://doi.org/10.1007/s12015-021-10172-4
Egorikhina MN, Rubtsova YP, Charykova IN, Bugrova ML, Bronnikova II, Mukhina PA, Sosnina LN, Aleynik DY (2020) Biopolymer hydrogel scaffold as an artificial cell niche for mesenchymal stem cells. Polymers (Basel) 12:2550. https://doi.org/10.3390/polym12112550
Lee EJ, Park SJ, Kang SK, Kim GH, Kang HJ, Lee SW, Jeon HB, Kim HS (2012) spherical bullet formation via e-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther 20:1424–1433. https://doi.org/10.1038/mt.2012.58
Qazi TH, Mooney DJ, Duda GN, Geissler S (2017) Biomaterials that promote cell-cell interactions enhance the paracrine function of Mscs. Biomaterials 140:103–114. https://doi.org/10.1016/j.biomaterials.2017.06.019
Bou-Ghannam S, Kim K, Grainger DW, Okano T (2021) 3d cell sheet structure augments mesenchymal stem cell cytokine production. Sci Rep 11:8170. https://doi.org/10.1038/s41598-021-87571-7
Campos F, Bonhome-Espinosa AB, Chato-Astrain J, Sanchez-Porras D, Garcia-Garcia OD, Carmona R, Lopez-Lopez MT, Alaminos M, Carriel V, Rodriguez IA (2020) Evaluation of fibrin-agarose tissue-like hydrogels biocompatibility for tissue engineering applications. Front Bioeng Biotechnol 8:596. https://doi.org/10.3389/fbioe.2020.00596
de Melo BAG, Jodat YA, Cruz EM, Benincasa JC, Shin SR, Porcionatto MA (2020) Strategies to use fibrinogen as bioink for 3d bioprinting fibrin-based soft and hard tissues. Acta Biomater 117:60–76. https://doi.org/10.1016/j.actbio.2020.09.024
Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B (2006) Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng 12:2385–2396. https://doi.org/10.1089/ten.2006.12.2385
Ho W, Tawil B, Dunn JC, Wu BM (2006) The behavior of human mesenchymal stem cells in 3d fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng 12:1587–1595. https://doi.org/10.1089/ten.2006.12.1587
Wang O, Ismail A, Fabian FM, Lin H, Li Q, Elowsky C, Carlson MA, Burgess W, Velander WH, Kidambi S, Lei Y (2018) A totally recombinant fibrin matrix for mesenchymal stem cell culture and delivery. J Biomed Mater Res A 106:3135–3142. https://doi.org/10.1002/jbm.a.36508
Mendez JJ, Ghaedi M, Sivarapatna A, Dimitrievska S, Shao Z, Osuji CO, Steinbacher DM, Leffell DJ, Niklason LE (2015) Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials 40:61–71. https://doi.org/10.1016/j.biomaterials.2014.11.011
Tan J, Li L, Wang H, Wei L, Gao X, Zeng Z, Liu S, Fan Y, Liu T, Chen J (2021) Biofunctionalized fibrin gel co-embedded with Bmscs and Vegf for accelerating skin injury repair. Mater Sci Eng C Mater Biol Appl 121:111749. https://doi.org/10.1016/j.msec.2020.111749
Carter K, Lee HJ, Na KS, Fernandes-Cunha GM, Blanco IJ, Djalilian A, Myung D (2019) Characterizing the impact of 2d and 3d culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater 99:247–257. https://doi.org/10.1016/j.actbio.2019.09.022
Acknowledgements
We thank Medjaden Inc. for scientific editing of this manuscript. We thank Figdraw (www.figdraw.com) for its help in creating the figures.
Funding
This study was supported by the National Key Research and Development Program of China (2022YFA1105600), Science and Technology Project of Wuhan (No: 2020020602012112), Haihe Laboratory of Cell Ecosystem Innovation Fund (HH22KYZX0046), and the Tianjin Free Trade Zone Innovation Development Project 211 (ZMCY-03-2021002-01) funded the study. We are also grateful for the support from the 3551 Talent Plan of China Optics Valley.
Author information
Authors and Affiliations
Contributions
YW performed literature reviewing and wrote the first draft of the manuscript. HD, TD, and LZ contributed partial literature study and discussion. WF, YZ, and WQ revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Dong, H., Dong, T. et al. Treatment of cytokine release syndrome-induced vascular endothelial injury using mesenchymal stem cells. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04785-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04785-1