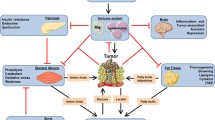
Abstract
Cancer cachexia can be defined as a complex metabolic syndrome characterized by weight loss, anorexia, and emaciation due to the wasting of adipose tissue and skeletal muscle. In the last decade, much research has been done to decipher the role of lipid metabolism in cancer cachexia. Tumors, as well as host-derived factors, cause major metabolic changes in the body. Metabolic changes lead to higher energy expenditure by the host. To meet the high energy demand, the host utilizes fat depots stored in adipose tissues by a process known as lipolysis. High catabolic and low anabolic response leads to loss of adipose tissue. A significant insight has been made regarding adipose tissue "browning" bestow on thermogenic activities of adipocytes that result in catabolic energy expenditure. Both lipolysis and WAT browning play an important role in exhaustion adipose tissue. The goal of this review is to summarise what is currently known and about altered lipid metabolism and its utilization in cancer cachexia.


Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- ACTH:
-
Adrenocorticotrophic hormone
- AT:
-
Adipose tissue
- AMPK:
-
AMP-activated protein kinase
- ATGL:
-
Adipose triglyceride lipase
- BAT:
-
Brown adipose tissue
- β1-AR:
-
β1 Adrenergic receptor
- DAG:
-
Diacylglycerol
- ERK:
-
Extracellular signal-regulated kinase
- FFA:
-
Free fatty acid
- FDG:
-
Fluorodeoxyglucose
- GPLR:
-
G protein-linked receptors
- GSK-3b:
-
Glycogen synthase kinase 3b
- HSL:
-
Hormone sensitive lipase
- IL-6:
-
Intraleukin-6
- LDL:
-
Low density protein
- LPL:
-
Lipoprotein lipase
- LIF:
-
Leukemia inhibitory factor
- MAPK:
-
P42/44mitogen-activated protein kinase
- MAG:
-
Monoacylglycerol
- PTHrP:
-
Parathyroid hormone-related peptide
- PET:
-
Positron emission tomography
- PGE:
-
Prostaglandin E
- PKA:
-
Protein kinase A
- Prdm 16:
-
PR domain containing 16
- Ppar:
-
Peroxisome proliferator-activated receptor
- PUFA:
-
Polyunsaturated fatty acid
- REE:
-
Resting energy expenditure
- SFA:
-
Saturated fatty acid
- TAG:
-
Triacylglycerol
- TNF-α:
-
Tumor necrosis factor-α
- TLR:
-
Toll like receptor
- UCP1:
-
Uncoupling protein 1
- VLDL:
-
Very low density protein
- ZAG:
-
Zinc-α2 -glycoprotein
References
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495. https://doi.org/10.1016/S1470-2045(10)70218-7
Anker MS, Holcomb R, Muscaritoli M, von Haehling S, Haverkamp W, Jatoi A, Morley JE, Strasser F, Landmesser U, Coats AJS, Anker SD (2019) Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle 10:22–34. https://doi.org/10.1002/jcsm.12402
Esper DH, Harb WA (2005) The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract 20:369–376. https://doi.org/10.1177/0115426505020004369
Petruzzelli M, Wagner EF (2016) Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev 30:489–501. https://doi.org/10.1101/gad.276733.115
Porporato PE (2016) Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 5:e200. https://doi.org/10.1038/oncsis.2016.3
Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, Kaasa S, Fearon K, Strasser F, European Palliative Care Research C (2011) Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol Hematol 80:114–144. https://doi.org/10.1016/j.critrevonc.2010.10.004
Weyandt JD, Thompson CB, Giaccia AJ, Rathmell WK (2017) Metabolic alterations in cancer and their potential as therapeutic targets. Am Soc Clin Oncol Educ Book 37:825–832. https://doi.org/10.14694/EDBK_175561
Schcolnik-Cabrera A, Chavez-Blanco A, Dominguez-Gomez G, Duenas-Gonzalez A (2017) Understanding tumor anabolism and patient catabolism in cancer-associated cachexia. Am J Cancer Res 7:1107–1135
Cohen P, Spiegelman BM (2016) Cell biology of fat storage. Mol Biol Cell 27:2523–2527. https://doi.org/10.1091/mbc.E15-10-0749
Alves-Bezerra M, Cohen DE (2017) Triglyceride metabolism in the liver. Compr Physiol 8:1–8. https://doi.org/10.1002/cphy.c170012
Hellerstein MK (1999) De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr 53(Suppl 1):S53-65. https://doi.org/10.1038/sj.ejcn.1600744
Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS (2007) Regulation of lipolysis in adipocytes. Annu Rev Nutr 27:79–101. https://doi.org/10.1146/annurev.nutr.27.061406.093734
Jensen MD, Ekberg K, Landau BR (2001) Lipid metabolism during fasting. Am J Physiol Endocrinol Metab 281:E789–E793. https://doi.org/10.1152/ajpendo.2001.281.4.E789
Tornqvist H, Belfrage P (1976) Purification and some properties of a monoacylglycerol-hydrolyzing enzyme of rat adipose tissue. J Biol Chem 251:813–819
Rohm M, Zeigerer A, Machado J, Herzig S (2019) Energy metabolism in cachexia. EMBO Rep. https://doi.org/10.15252/embr.201847258
Fearon KC, Glass DJ, Guttridge DC (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16:153–166. https://doi.org/10.1016/j.cmet.2012.06.011
Sidossis L, Kajimura S (2015) Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125:478–486. https://doi.org/10.1172/JCI78362
Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN (2015) Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 22:219–227. https://doi.org/10.1016/j.cmet.2015.06.022
Tisdale MJ (2009) Mechanisms of cancer cachexia. Physiol Rev 89:381–410. https://doi.org/10.1152/physrev.00016.2008
Argiles JM, Alvarez B, Lopez-Soriano FJ (1997) The metabolic basis of cancer cachexia. Med Res Rev 17:477–498. https://doi.org/10.1002/(sici)1098-1128(199709)17:5%3c477::aid-med3%3e3.0.co;2-R
Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G (2011) Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333:233–238. https://doi.org/10.1126/science.1198973
Costelli P, Tessitore L, Batetta B, Mulas MF, Spano O, Pani P, Baccino FM, Dessi S (1999) Alterations of lipid and cholesterol metabolism in cachectic tumor-bearing rats are prevented by insulin. J Nutr 129:700–706. https://doi.org/10.1093/jn/129.3.700
Riccardi D, das Neves RX, de Matos-Neto EM, Camargo RG, Lima J, Radloff K, Alves MJ, Costa RGF, Tokeshi F, Otoch JP, Maximiano LF, de Alcantara PSM, Colquhoun A, Laviano A, Seelaender M (2020) Plasma lipid profile and systemic inflammation in patients with cancer cachexia. Front Nutr 7:4. https://doi.org/10.3389/fnut.2020.00004
Zwickl H, Hackner K, Kofeler H, Krzizek EC, Muqaku B, Pils D, Scharnagl H, Solheim TS, Zwickl-Traxler E, Pecherstorfer M (2020) Reduced LDL-cholesterol and reduced total cholesterol as potential indicators of early cancer in male treatment-naive cancer patients with pre-cachexia and cachexia. Front Oncol 10:1262. https://doi.org/10.3389/fonc.2020.01262
Korber J, Pricelius S, Heidrich M, Muller MJ (1999) Increased lipid utilization in weight losing and weight stable cancer patients with normal body weight. Eur J Clin Nutr 53:740–745. https://doi.org/10.1038/sj.ejcn.1600843
Tisdale MJ (2010) Are tumoral factors responsible for host tissue wasting in cancer cachexia? Future Oncol 6:503–513. https://doi.org/10.2217/fon.10.20
Das SK, Hoefler G (2013) The role of triglyceride lipases in cancer associated cachexia. Trends Mol Med 19:292–301. https://doi.org/10.1016/j.molmed.2013.02.006
Greenberg AS, Nordan RP, McIntosh J, Calvo JC, Scow RO, Jablons D (1992) Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res 52:4113–4116
Nomura K, Noguchi Y, Yoshikawa T, Kondo J (1997) Plasma interleukin-6 is not a mediator of changes in lipoprotein lipase activity in cancer patients. Hepatogastroenterology 44:1519–1526
Kawamura I, Yamamoto N, Sakai F, Yamazaki H, Goto T (1999) Effect of lipoprotein lipase activators bezafibrate and NO-1886, on B16 melanoma-induced cachexia in mice. Anticancer Res 19:4099–4103
Briddon S, Beck SA, Tisdale MJ (1991) Changes in activity of lipoprotein lipase, plasma free fatty acids and triglycerides with weight loss in a cachexia model. Cancer Lett 57:49–53. https://doi.org/10.1016/0304-3835(91)90062-m
Tsujimoto S, Kawamura I, Inami M, Lacey E, Nishigaki F, Naoe Y, Manda T, Goto T (2000) Cachexia induction by EL-4 lymphoma in mice and possible involvement of impaired lipoprotein lipase activity. Anticancer Res 20:3111–3116
Kawakami M, Kondo Y, Imai Y, Hashiguchi M, Ogawa H, Hiragun A, Aotsuka S, Shibata S, Oda T, Murase T et al (1991) Suppression of lipoprotein lipase in 3T3-L1 cells by a mediator produced by SEKI melanoma, a cachexia-inducing human melanoma cell line. J Biochem 109:78–82
Berg M, Fraker DL, Alexander HR (1994) Characterization of differentiation factor/leukaemia inhibitory factor effect on lipoprotein lipase activity and mRNA in 3T3-L1 adipocytes. Cytokine 6:425–432. https://doi.org/10.1016/1043-4666(94)90067-1
Nara-Ashizawa N, Akiyama Y, Maruyama K, Tsukada T, Yamaguchi K (2001) Lipolytic and lipoprotein lipase (LPL)-inhibiting activities produced by a human lung cancer cell line responsible for cachexia induction. Anticancer Res 21:3381–3387
Kawamura I, Lacey E, Yamamoto N, Sakai F, Takeshita S, Inami M, Nishigaki F, Naoe Y, Tsujimoto S, Manda T, Shimomura K, Goto T (1999) Ponalrestat, an aldose reductase inhibitor, inhibits cachexia syndrome induced by colon26 adenocarcinoma in mice. Anticancer Res 19:4105–4111
Nomura K, Noguchi Y, Matsumoto A (1996) Stimulation of decreased lipoprotein lipase activity in the tumor-bearing state by the antihyperlipidemic drug bezafibrate. Surg Today 26:89–94. https://doi.org/10.1007/BF00311770
Vaughan M, Berger JE, Steinberg D (1964) Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. J Biol Chem 239:401–409
Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F (2012) FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab 15:279–291. https://doi.org/10.1016/j.cmet.2011.12.018
Miyoshi H, Perfield JW 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS (2007) Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem 282:996–1002. https://doi.org/10.1074/jbc.M605770200
Cao DX, Wu GH, Yang ZA, Zhang B, Jiang Y, Han YS, He GD, Zhuang QL, Wang YF, Huang ZL, Xi QL (2010) Role of beta1-adrenoceptor in increased lipolysis in cancer cachexia. Cancer Sci 101:1639–1645. https://doi.org/10.1111/j.1349-7006.2010.01582.x
Agustsson T, Ryden M, Hoffstedt J, van Harmelen V, Dicker A, Laurencikiene J, Isaksson B, Permert J, Arner P (2007) Mechanism of increased lipolysis in cancer cachexia. Cancer Res 67:5531–5537. https://doi.org/10.1158/0008-5472.CAN-06-4585
Yang X, Zhang X, Heckmann BL, Lu X, Liu J (2011) Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-alpha (TNF-alpha)-induced lipolysis in adipocytes. J Biol Chem 286:40477–40485. https://doi.org/10.1074/jbc.M111.257923
Borin TF, Angara K, Rashid MH, Achyut BR, Arbab AS (2017) Arachidonic acid metabolite as a novel therapeutic target in breast cancer metastasis. Int J Mol Sci. https://doi.org/10.3390/ijms18122661
Wang W, Andersson M, Lonnroth C, Svanberg E, Lundholm K (2005) Prostaglandin E and prostacyclin receptor expression in tumor and host tissues from MCG 101-bearing mice: a model with prostanoid-related cachexia. Int J Cancer 115:582–590. https://doi.org/10.1002/ijc.20539
Lai V, George J, Richey L, Kim HJ, Cannon T, Shores C, Couch M (2008) Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck 30:67–74. https://doi.org/10.1002/hed.20662
Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ (2004) Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53:25–31. https://doi.org/10.2337/diabetes.53.1.25
Morigny P, Zuber J, Haid M, Kaltenecker D, Riols F, Lima JDC, Simoes E, Otoch JP, Schmidt SF, Herzig S, Adamski J, Seelaender M, Berriel Diaz M, Rohm M (2020) High levels of modified ceramides are a defining feature of murine and human cancer cachexia. J Cachexia Sarcopenia Muscle 11:1459–1475. https://doi.org/10.1002/jcsm.12626
Sokolowska E, Blachnio-Zabielska A (2019) The role of ceramides in insulin resistance. Front Endocrinol (Lausanne) 10:577. https://doi.org/10.3389/fendo.2019.00577
Shahidi F, Ambigaipalan P (2018) Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol 9:345–381. https://doi.org/10.1146/annurev-food-111317-095850
Tisdale MJ (1993) Mechanism of lipid mobilization associated with cancer cachexia: interaction between the polyunsaturated fatty acid, eicosapentaenoic acid, and inhibitory guanine nucleotide-regulatory protein. Prostaglandins Leukot Essent Fatty Acids 48:105–109. https://doi.org/10.1016/0952-3278(93)90017-q
Gu Z, Shan K, Chen H, Chen YQ (2015) n-3 polyunsaturated fatty acids and their role in cancer chemoprevention. Curr Pharmacol Rep 1:283–294. https://doi.org/10.1007/s40495-015-0043-9
Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ (2003) NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol 284:L84–L89. https://doi.org/10.1152/ajplung.00077.2002
Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ (2001) Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 61:3604–3609
Whitehouse AS, Tisdale MJ (2003) Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-kappaB. Br J Cancer 89:1116–1122. https://doi.org/10.1038/sj.bjc.6601132
Sauer LA, Dauchy RT, Blask DE (2000) Mechanism for the antitumor and anticachectic effects of n-3 fatty acids. Cancer Res 60:5289–5295
Islam-Ali B, Khan S, Price SA, Tisdale MJ (2001) Modulation of adipocyte G-protein expression in cancer cachexia by a lipid-mobilizing factor (LMF). Br J Cancer 85:758–763. https://doi.org/10.1054/bjoc.2001.1992
Abe K, Uwagawa T, Haruki K, Takano Y, Onda S, Sakamoto T, Gocho T, Yanaga K (2018) Effects of omega-3 fatty acid supplementation in patients with bile duct or pancreatic cancer undergoing chemotherapy. Anticancer Res 38:2369–2375. https://doi.org/10.21873/anticanres.12485
Mocellin MC, Fernandes R, Chagas TR, Trindade E (2018) A meta-analysis of n-3 polyunsaturated fatty acids effects on circulating acute-phase protein and cytokines in gastric cancer. Clin Nutr 37:840–850. https://doi.org/10.1016/j.clnu.2017.05.008
Cerchietti LC, Navigante AH, Castro MA (2007) Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer 59:14–20. https://doi.org/10.1080/01635580701365068
Gorjao R, Dos Santos CMM, Serdan TDA, Diniz VLS, Alba-Loureiro TC, Cury-Boaventura MF, Hatanaka E, Levada-Pires AC, Sato FT, Pithon-Curi TC, Fernandes LC, Curi R, Hirabara SM (2019) New insights on the regulation of cancer cachexia by N-3 polyunsaturated fatty acids. Pharmacol Ther 196:117–134. https://doi.org/10.1016/j.pharmthera.2018.12.001
Beutler B, Mahoney J, Le Trang N, Pekala P, Cerami A (1985) Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med 161:984–995. https://doi.org/10.1084/jem.161.5.984
Cornelius P, Enerback S, Bjursell G, Olivecrona T, Pekala PH (1988) Regulation of lipoprotein lipase mRNA content in 3T3-L1 cells by tumour necrosis factor. Biochem J 249:765–769. https://doi.org/10.1042/bj2490765
Chen SZ, Qiu ZG (2011) Combined treatment with GH, insulin, and indomethacin alleviates cancer cachexia in a mouse model. J Endocrinol 208:131–136. https://doi.org/10.1677/JOE-10-0341
Sherry BA, Gelin J, Fong Y, Marano M, Wei H, Cerami A, Lowry SF, Lundholm KG, Moldawer LL (1989) Anticachectin/tumor necrosis factor-alpha antibodies attenuate development of cachexia in tumor models. FASEB J 3:1956–1962. https://doi.org/10.1096/fasebj.3.8.2721856
Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, Rodriguez A (2014) Regulation of adipocyte lipolysis. Nutr Res Rev 27:63–93. https://doi.org/10.1017/S095442241400002X
Langin D, Arner P (2006) Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab 17:314–320. https://doi.org/10.1016/j.tem.2006.08.003
Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF (2002) Tumor necrosis factor-alpha suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by TNF-alpha is obligatory. Diabetes 51:1319–1336. https://doi.org/10.2337/diabetes.51.5.1319
Souza SC, de Vargas LM, Yamamoto MT, Lien P, Franciosa MD, Moss LG, Greenberg AS (1998) Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes. J Biol Chem 273:24665–24669. https://doi.org/10.1074/jbc.273.38.24665
Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C (2004) The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 56:379–385. https://doi.org/10.1080/15216540400009968
Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, Mattar BI, Loprinzi CL (2010) A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 68:234–239. https://doi.org/10.1016/j.lungcan.2009.06.020
Diez-Itza I, Sanchez LM, Allende MT, Vizoso F, Ruibal A, Lopez-Otin C (1993) Zn-alpha 2-glycoprotein levels in breast cancer cytosols and correlation with clinical, histological and biochemical parameters. Eur J Cancer 29A:1256–1260. https://doi.org/10.1016/0959-8049(93)90068-q
Bondar OP, Barnidge DR, Klee EW, Davis BJ, Klee GG (2007) LC-MS/MS quantification of Zn-alpha2 glycoprotein: a potential serum biomarker for prostate cancer. Clin Chem 53:673–678. https://doi.org/10.1373/clinchem.2006.079681
Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P (2004) Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 101:2500–2505. https://doi.org/10.1073/pnas.0308647100
Satyanarayana A, Klarmann KD, Gavrilova O, Keller JR (2012) Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet induced insulin resistance, and hepatosteatosis. FASEB J 26:309–323. https://doi.org/10.1096/fj.11-190892
Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896. https://doi.org/10.1038/nrm2066
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM (2002) C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 16:22–26. https://doi.org/10.1101/gad.948702
Weidle UH, Klostermann S, Eggle D, Kruger A (2010) Interleukin 6/interleukin 6 receptor interaction and its role as a therapeutic target for treatment of cachexia and cancer. Cancer Genom Proteom 7:287–302
Tsoli M, Moore M, Burg D, Painter A, Taylor R, Lockie SH, Turner N, Warren A, Cooney G, Oldfield B, Clarke S, Robertson G (2012) Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res 72:4372–4382. https://doi.org/10.1158/0008-5472.CAN-11-3536
Inadera H, Nagai S, Dong HY, Matsushima K (2002) Molecular analysis of lipid-depleting factor in a colon-26-inoculated cancer cachexia model. Int J Cancer 101:37–45. https://doi.org/10.1002/ijc.10578
Grant RW, Stephens JM (2015) Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. Am J Physiol Endocrinol Metab 309:E205–E213. https://doi.org/10.1152/ajpendo.00053.2015
Cannon TY, Guttridge D, Dahlman J, George JR, Lai V, Shores C, Buzkova P, Couch ME (2007) The effect of altered Toll-like receptor 4 signaling on cancer cachexia. Arch Otolaryngol Head Neck Surg 133:1263–1269. https://doi.org/10.1001/archotol.133.12.1263
Bohnert KR, Goli P, Roy A, Sharma AK, Xiong G, Gallot YS, Kumar A (2019) The toll-like receptor/MyD88/XBP1 signaling axis mediates skeletal muscle wasting during cancer cachexia. Mol Cell Biol. https://doi.org/10.1128/MCB.00184-19
Maccio A, Sanna E, Neri M, Oppi S, Madeddu C (2021) Cachexia as evidence of the mechanisms of resistance and tolerance during the evolution of cancer disease. Int J Mol Sci. https://doi.org/10.3390/ijms22062890
Henriques F, Lopes MA, Franco FO, Knobl P, Santos KB, Bueno LL, Correa VA, Bedard AH, Guilherme A, Birbrair A, Peres SB, Farmer SR, Batista ML Jr (2018) Toll-like receptor-4 disruption suppresses adipose tissue remodeling and increases survival in cancer cachexia syndrome. Sci Rep 8:18024. https://doi.org/10.1038/s41598-018-36626-3
Sin TK, Zhang G, Zhang Z, Gao S, Li M, Li YP (2019) Cancer takes a toll on skeletal muscle by releasing heat shock proteins-an emerging mechanism of cancer-induced cachexia. Cancers (Basel). https://doi.org/10.3390/cancers11091272
Ishiko O, Nishimura S, Yasui T, Sumi T, Hirai K, Honda K, Ogita S (1999) Metabolic and morphologic characteristics of adipose tissue associated with the growth of malignant tumors. Jpn J Cancer Res 90:655–659. https://doi.org/10.1111/j.1349-7006.1999.tb00797.x
Byerley LO, Lee SH, Redmann S, Culberson C, Clemens M, Lively MO (2010) Evidence for a novel serum factor distinct from zinc alpha-2 glycoprotein that promotes body fat loss early in the development of cachexia. Nutr Cancer 62:484–494. https://doi.org/10.1080/01635580903441220
Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG (2005) Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care–correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 103:2189–2198. https://doi.org/10.1002/cncr.21013
Murphy RA, Wilke MS, Perrine M, Pawlowicz M, Mourtzakis M, Lieffers JR, Maneshgar M, Bruera E, Clandinin MT, Baracos VE, Mazurak VC (2010) Loss of adipose tissue and plasma phospholipids: relationship to survival in advanced cancer patients. Clin Nutr 29:482–487. https://doi.org/10.1016/j.clnu.2009.11.006
Mracek T, Stephens NA, Gao D, Bao Y, Ross JA, Ryden M, Arner P, Trayhurn P, Fearon KC, Bing C (2011) Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br J Cancer 104:441–447. https://doi.org/10.1038/sj.bjc.6606083
Nordstrom EA, Ryden M, Backlund EC, Dahlman I, Kaaman M, Blomqvist L, Cannon B, Nedergaard J, Arner P (2005) A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-alpha)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes 54:1726–1734. https://doi.org/10.2337/diabetes.54.6.1726
Bing C, Russell S, Becket E, Pope M, Tisdale MJ, Trayhurn P, Jenkins JR (2006) Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br J Cancer 95:1028–1037. https://doi.org/10.1038/sj.bjc.6603360
Dahlman I, Mejhert N, Linder K, Agustsson T, Mutch DM, Kulyte A, Isaksson B, Permert J, Petrovic N, Nedergaard J, Sjolin E, Brodin D, Clement K, Dahlman-Wright K, Ryden M, Arner P (2010) Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer 102:1541–1548. https://doi.org/10.1038/sj.bjc.6605665
Ebadi M, Field CJ, Lehner R, Mazurak VC (2017) Chemotherapy diminishes lipid storage capacity of adipose tissue in a preclinical model of colon cancer. Lipids Health Dis 16:247. https://doi.org/10.1186/s12944-017-0638-8
Biondo LA, Lima Junior EA, Souza CO, Cruz MM, Cunha RD, Alonso-Vale MI, Oyama LM, Nascimento CM, Pimentel GD, Dos Santos RV, Lira FS, Rosa Neto JC (2016) Impact of doxorubicin treatment on the physiological functions of white adipose tissue. PLoS ONE 11:e0151548. https://doi.org/10.1371/journal.pone.0151548
Vergoni B, Cornejo PJ, Gilleron J, Djedaini M, Ceppo F, Jacquel A, Bouget G, Ginet C, Gonzalez T, Maillet J, Dhennin V, Verbanck M, Auberger P, Froguel P, Tanti JF, Cormont M (2016) DNA damage and the activation of the p53 pathway mediate alterations in metabolic and secretory functions of adipocytes. Diabetes 65:3062–3074. https://doi.org/10.2337/db16-0014
Biondo LA, Batatinha HA, Souza CO, Teixeira AAS, Silveira LS, Alonso-Vale MI, Oyama LM, Alves MJ, Seelaender M, Neto JCR (2018) Metformin mitigates fibrosis and glucose intolerance induced by doxorubicin in subcutaneous adipose tissue. Front Pharmacol 9:452. https://doi.org/10.3389/fphar.2018.00452
Sullivan ES, Daly LE, Bhuachalla ÉB, Cushen SJ, Ryan AM, Power DG (2019) Loss of subcutaneous adipose tissue during chemotherapy predicts reduced survival in patients with incurable colorectal cancer undergoing palliative therapy. Ann Oncol. https://doi.org/10.1093/annonc/mdz246.127
Brendle C, Stefan N, Stef I, Ripkens S, Soekler M, la Fougere C, Nikolaou K, Pfannenberg C (2019) Impact of diverse chemotherapeutic agents and external factors on activation of brown adipose tissue in a large patient collective. Sci Rep 9:1901. https://doi.org/10.1038/s41598-018-37924-6
Shellock FG, Riedinger MS, Fishbein MC (1986) Brown adipose tissue in cancer patients: possible cause of cancer-induced cachexia. J Cancer Res Clin Oncol 111:82–85. https://doi.org/10.1007/BF00402783
Sun X, Feng X, Wu X, Lu Y, Chen K, Ye Y (2020) Fat wasting is damaging: role of adipose tissue in cancer-associated cachexia. Front Cell Dev Biol 8:33. https://doi.org/10.3389/fcell.2020.00033
Townsend K, Tseng YH (2012) Brown adipose tissue: recent insights into development, metabolic function and therapeutic potential. Adipocyte 1:13–24. https://doi.org/10.4161/adip.18951
Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A (2011) Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480:104–108. https://doi.org/10.1038/nature10653
Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293:E444–E452. https://doi.org/10.1152/ajpendo.00691.2006
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508. https://doi.org/10.1056/NEJMoa0808718
Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, Hodin RA, Spiegelman BM (2016) PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab 23:315–323. https://doi.org/10.1016/j.cmet.2015.11.003
Patsouris D, Qi P, Abdullahi A, Stanojcic M, Chen P, Parousis A, Amini-Nik S, Jeschke MG (2015) Burn induces browning of the subcutaneous white adipose tissue in mice and humans. Cell Rep 13:1538–1544. https://doi.org/10.1016/j.celrep.2015.10.028
Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, Zechner R, Wagner EF (2014) A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 20:433–447. https://doi.org/10.1016/j.cmet.2014.06.011
Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM (2014) Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513:100–104. https://doi.org/10.1038/nature13528
Wu Q, Sun S, Li Z, Yang Q, Li B, Zhu S, Wang L, Wu J, Yuan J, Wang C, Li J, Sun S (2019) Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression. Adipocyte 8:31–45. https://doi.org/10.1080/21623945.2018.1551688
Di W, Zhang W, Zhu B, Li X, Tang Q, Zhou Y (2021) Colorectal cancer prompted adipose tissue browning and cancer cachexia through transferring exosomal miR-146b-5p. J Cell Physiol 236:5399–5410. https://doi.org/10.1002/jcp.30245
Zhang H, Zhu L, Bai M, Liu Y, Zhan Y, Deng T, Yang H, Sun W, Wang X, Zhu K, Fan Q, Li J, Ying G, Ba Y (2019) Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int J Cancer 144:2501–2515. https://doi.org/10.1002/ijc.31977
Wang H, Zheng Q, Lu Z, Wang L, Ding L, Xia L, Zhang H, Wang M, Chen Y, Li G (2021) Role of the nervous system in cancers: a review. Cell Death Discov 7:76. https://doi.org/10.1038/s41420-021-00450-y
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19:55–71. https://doi.org/10.1038/s41579-020-0433-9
Bindels LB, Neyrinck AM, Claus SP, Le Roy CI, Grangette C, Pot B, Martinez I, Walter J, Cani PD, Delzenne NM (2016) Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J 10:1456–1470. https://doi.org/10.1038/ismej.2015.209
Bindels LB, Neyrinck AM, Loumaye A, Catry E, Walgrave H, Cherbuy C, Leclercq S, Van Hul M, Plovier H, Pachikian B, Bermudez-Humaran LG, Langella P, Cani PD, Thissen JP, Delzenne NM (2018) Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget 9:18224–18238. https://doi.org/10.18632/oncotarget.24804
Ubachs J, Ziemons J, Soons Z, Aarnoutse R, van Dijk DPJ, Penders J, van Helvoort A, Smidt ML, Kruitwagen R, Baade-Corpelijn L, Olde Damink SWM, Rensen SS (2021) Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.12804
Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, Ilievska M, Montet X, Seimbille Y, Hapfelmeier S, Trajkovski M (2015) Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med 21:1497–1501. https://doi.org/10.1038/nm.3994
Tsoli M, Robertson G (2013) Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol Metab 24:174–183. https://doi.org/10.1016/j.tem.2012.10.006
Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, Brans B (2014) Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging 41:776–791. https://doi.org/10.1007/s00259-013-2611-8
Acknowledgement
This manuscript is a part of PhD thesis of Mit Joshi, to be submitted to Nirma University, Ahmedabad, India and authors are thankful to Nirma University for providing required support for the same.
Funding
No funding was received for the current work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joshi, M., Patel, B.M. The burning furnace: Alteration in lipid metabolism in cancer-associated cachexia. Mol Cell Biochem 477, 1709–1723 (2022). https://doi.org/10.1007/s11010-022-04398-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04398-0