Abstract
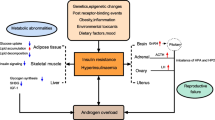
Since the lack of certainty in identifying polycystic ovary syndrome (PCOS) demonstrates confusion regarding the disorder’s pathophysiology and its therapeutic approaches, systematic screening of women under diagnostic guidelines of the NIH reported that about 4–10 percent of reproductive women aged 20–44 years suffer from PCOS. Not all females with PCOS-defining biochemical and clinical characteristics and about 22% of PCOS women have no symptoms. PCOS is a heterogeneous phenotypic and clinical condition, combined with metabolic implications. The root cause of PCOS is the major issue of IR or irregular androgen secretion and constant effort is being made in identifying the dynamic pathogenic network underlying the syndrome. Regardless of PCOS initiating cause, IR therapy and hyperinsulinemia can restore metabolic and hormonal homeostasis, and minimize ovarian dysfunction. Thus, the impact of insulin on ovaries in hyperinsulinemic individuals can account for many of the PCOS characteristics and is important for developing treatment strategies. Therefore, our primary aim is to investigate the proper understanding of endocrine disruption during PCOS and secondary to the therapeutic potential of inositol in reestablishing the equilibrium of ovarian dysfunction, anovulation, and eventually infertility.


Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Stein IF, Leventhal ML (1935) Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 29:181–191. https://doi.org/10.1016/S0002-9378(15)30642-6
Ahmad A, Gupta G, Afzal M, Kazmi I, Anwar F (2013) Antiulcer and antioxidant activities of a new steroid from Morus alba. Life Sci 92:202–210
Evans TN, Riley GM (1958) Polycystic ovarian disease (Stein-Leventhal syndrome); etiology and rationale for surgical treatment. Obstet Gynecol 12:168–179
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749. https://doi.org/10.1210/jc.2003-032046
Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47. https://doi.org/10.1093/humrep/deh098
Dokras A (2012) Mood and anxiety disorders in women with PCOS. Steroids 77:338–341. https://doi.org/10.1016/j.steroids.2011.12.008
Boyle J, Teede H (2012) Polycystic ovary syndrome an update. Aust Fam Physician 41:752–756
Nestler JE, Unfer V (2015) Reflections on inositol(s) for PCOS therapy: steps toward success. Gynecol Endocrinol 31:501–505. https://doi.org/10.3109/09513590.2015.1054802
Apridonidze T, Essah PA, Iuorno MJ, Nestler JE (2005) Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:1929–1935. https://doi.org/10.1210/jc.2004-1045
Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS (1998) Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clin Endocrinol (Oxf) 49:91–99. https://doi.org/10.1046/j.1365-2265.1998.00492.x
Gupta G, Krishna G, Chellappan DK, Gubbiyappa KS, Candasamy M, Dua K (2014) Protective effect of pioglitazone, a PPARγ agonist against acetaminophen-induced hepatotoxicity in rats. Mol Cell Biochem 393:223–228
Rawat S, Gupta G, Pathak S, Singh SK, Singh H, Mishra A, Gilhotra R, Aljabali AA, Dureja H, Tambuwala MM (2020) Current biological and pharmacological updates on wogonin. EXCLI J 19:635–640
El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G (2016) Poly cystic ovarian syndrome: an updated overview. Front Physiol 7:124–124. https://doi.org/10.3389/fphys.2016.00124
Escobar-Morreale HF (2018) Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 14:270–284. https://doi.org/10.1038/nrendo.2018.24
Balen AH, Rutherford AJ (2007) Managing anovulatory infertility and polycystic ovary syndrome. BMJ 335:663–666. https://doi.org/10.1136/bmj.39335.462303.80
Gupta G, Kazmi I, Afzal M, Rahman M, Saleem S, Ashraf MS, Khusroo MJ, Nazeer K, Ahmed S, Mujeeb M (2012) Sedative, antiepileptic and antipsychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J Ethnopharmacol 141:810–816
Samuel VP, Dahiya R, Singh Y, Gupta G, Sah SK, Gubbiyappa SK, Chellappan DK, Dua K (2019) Metformin: a salutary candidate for colorectal cancer treatment in patients with diabetes. J Environ Pathol Toxicol Oncol 38:133–141
Lim SS, Davies MJ, Norman RJ, Moran LJ (2012) Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 18:618–637. https://doi.org/10.1093/humupd/dms030
Papadakis G, Kandaraki E, Papalou O, Vryonidou A, Diamanti-Kandarakis E (2017) Is cardiovascular risk in women with PCOS a real risk? Current insights. Minerva Endocrinol 42:340–355. https://doi.org/10.23736/s0391-1977.17.02609-8
Zumoff B, Freeman R, Coupey S, Saenger P, Markowitz M, Kream J (1983) A chronobiologic abnormality in luteinizing hormone secretion in teenage girls with the polycystic-ovary syndrome. N Engl J Med 309:1206–1209. https://doi.org/10.1056/nejm198311173092002
Gupta G, Singh R, David SR, Verma RK (2013) Effect of rosiglitazone, a PPAR-γ ligand on haloperidol-induced catalepsy. CNS Neurosci Ther 19:724
Hu S, Xu B, Long R, Jin L (2021) The effect of polycystic ovary syndrome (PCOS) without hyperandrogenism on pregnancy-related outcomes: a retrospective cohort study. BJOG: Int J Obstet Gynaecol. https://doi.org/10.1111/1471-0528.16557
Wang XX, Wei JZ, Jiao J, Jiang SY, Yu DH, Li D (2014) Genome-wide DNA methylation and gene expression patterns provide insight into polycystic ovary syndrome development. Oncotarget 5:6603–6610. https://doi.org/10.18632/oncotarget.2224
Marshall JC, Dunaif A (2012) Should all women with PCOS be treated for insulin resistance? Fertil Steril 97:18–22. https://doi.org/10.1016/j.fertnstert.2011.11.036
Pasquali R, Gambineri A (2013) Insulin sensitizers in polycystic ovary syndrome. Front Horm Res 40:83–102. https://doi.org/10.1159/000341837
Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI (2017) Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 23:390–408. https://doi.org/10.1093/humupd/dmx012
Gupta G, Singh Y, Tiwari J, Raizaday A, Alharbi KS, Al-Abbasi FA, Kazmi I, Satija S, Tambuwala MM, Devkota HP (2020) Beta-catenin non-canonical pathway: a potential target for inflammatory and hyperproliferative state via expression of transglutaminase 2 in psoriatic skin keratinocyte. Dermatol Ther 33:e14209
Singh Y, Samuel VP, Dahiya S, Gupta G, Gillhotra R, Mishra A, Singh M, SreeHarsha N, Gubbiyappa SK, Tambuwala MM (2019) Combinational effect of angiotensin receptor blocker and folic acid therapy on uric acid and creatinine level in hyperhomocysteinemia-associated hypertension. Biotechnol Appl Biochem 66:715–719
Macut D, Bjekić-Macut J, Rahelić D, Doknić M (2017) Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract 130:163–170. https://doi.org/10.1016/j.diabres.2017.06.011
Christian CA, Moenter SM (2010) The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev 31:544–577. https://doi.org/10.1210/er.2009-0023
McArdle C, Perrett R (2013) Molecular mechanisms of gonadotropin-releasing hormone signaling: integrating cyclic nucleotides into the network. Front Endocrinol. https://doi.org/10.3389/fendo.2013.00180
Herbison AE (2014) Physiology of the adult gonadotropin-releasing hormone neuronal network. In: Zeleznik TPA (ed) In Knobil and Neill’s physiology of reproduction, 4th edn. Elsevier Inc, San Diego, pp 399–467
Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F (1976) Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 57:1320–1329. https://doi.org/10.1172/jci108400
Qin KN, Rosenfield RL (1998) Role of cytochrome P450c17 in polycystic ovary syndrome. Mol Cell Endocrinol 145:111–121. https://doi.org/10.1016/s0303-7207(98)00177-4
Fröjdö S, Vidal H, Pirola L (2009) Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochimica et Biophysica Acta (BBA) 1792:83–92. https://doi.org/10.1016/j.bbadis.2008.10.019
Dunaif A, Xia J, Book CB, Schenker E, Tang Z (1995) Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest 96:801–810. https://doi.org/10.1172/jci118126
Gupta G, Afzal M, David SR, Verma R, Candaswamy M, Anwar F (2014) Anticonvulsant activity of Morus alba and its effect on brain gamma-aminobutyric acid level in rats. Pharmacognosy research 6:188
Tiwari J, Gupta G, deJesusAndreoliPinto T, Sharma R, Pabreja K, Matta Y, Arora N, Mishra A, Dua K (2018) Role of microRNAs (miRNAs) in the pathophysiology of diabetes mellitus. Panminerva Med. https://doi.org/10.23736/S0031-0808.17.03382-1
Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, Gorden P (2004) Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 83:209–222. https://doi.org/10.1097/01.md.0000133625.73570.54
Xiong Y-l, Liang X-y, Yang X, Li Y, Wei L-n (2011) Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol 159:148–150. https://doi.org/10.1016/j.ejogrb.2011.07.012
Escobar-Morreale HF, Calvo RM, Sancho J, San Millán JL (2001) TNF-alpha and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab 86:3761–3767. https://doi.org/10.1210/jcem.86.8.7770
Kelly CJ, Stenton SR, Lashen H (2011) Insulin-like growth factor binding protein-1 in PCOS: a systematic review and meta-analysis. Hum Reprod Update 17:4–16. https://doi.org/10.1093/humupd/dmq027
Poretsky L (1991) On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev 12:3–13. https://doi.org/10.1210/edrv-12-1-3
Plymate SR, Matej LA, Jones RE, Friedl KE (1988) Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67:460–464. https://doi.org/10.1210/jcem-67-3-460
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R (2002) Obesity and the polycystic ovary syndrome. Int J Obes 26:883–896. https://doi.org/10.1038/sj.ijo.0801994
Kazmi I, Rahman M, Afzal M, Gupta G, Saleem S, Afzal O, Shaharyar MA, Nautiyal U, Ahmed S, Anwar F (2012) Anti-diabetic potential of ursolic acid stearoyl glucoside: a new triterpenic gycosidic ester from Lantana camara. Fitoterapia 83:142–146
Rahman M, Zaki Ahmad M, Kazmi I, Akhter S, Afzal M, Gupta G, Ranjan Sinha V (2012) Emergence of nanomedicine as cancer targeted magic bullets: recent development and need to address the toxicity apprehension. Curr Drug Discov Technol 9:319–329
Greene DA, De JesusWinegrad PVAI Jr (1975) Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest 55:1326–1336. https://doi.org/10.1172/JCI108052
Laganà AS, Garzon S, Casarin J, Franchi M, Ghezzi F (2018) Inositol in polycystic ovary syndrome: restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab 29:768–780. https://doi.org/10.1016/j.tem.2018.09.001
Larner J (2002) d-chiro-inositol—Its functional role in insulin action and its deficit in insulin resistance. Int J Exp Diabetes Res 3:647841. https://doi.org/10.1080/15604280212528
Dinicola S, Chiu TT, Unfer V, Carlomagno G, Bizzarri M (2014) The rationale of the myo-inositol and D-chiro-inositol combined treatment for polycystic ovary syndrome. J Clin Pharmacol 54:1079–1092. https://doi.org/10.1002/jcph.362
Monastra G, Unfer V, Harrath AH, Bizzarri M (2017) Combining treatment with myo-inositol and D-chiro-inositol (40:1) is effective in restoring ovary function and metabolic balance in PCOS patients. Gynecol Endocrinol 33:1–9. https://doi.org/10.1080/09513590.2016.1247797
Paul C, Laganà AS, Maniglio P, Triolo O, Brady DM (2016) Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecol Endocrinol 32:431–438. https://doi.org/10.3109/09513590.2016.1144741
Unfer V, Nestler JE, Kamenov ZA, Prapas N, Facchinetti F (2016) Effects of inositol(s) in women with PCOS: a systematic review of randomized controlled trials. Int J Endocrinol 2016:1849162. https://doi.org/10.1155/2016/1849162
Larner J (2002) D-chiro-inositol–its functional role in insulin action and its deficit in insulin resistance. Int J Exp Diabetes Res 3:47–60. https://doi.org/10.1080/15604280212528
Sacchi S, Marinaro F, Tondelli D, Lui J, Xella S, Marsella T, Tagliasacchi D, Argento C, Tirelli A, Giulini S, La Marca A (2016) Modulation of gonadotrophin induced steroidogenic enzymes in granulosa cells by D-chiro-inositol. Reprod Biol Endocrinol 14:52. https://doi.org/10.1186/s12958-016-0189-2
Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F (1998) Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83:2001–2005. https://doi.org/10.1210/jcem.83.6.4886
Milewska EM, Czyzyk A, Meczekalski B, Genazzani AD (2016) Inositol and human reproduction. From cellular metabolism to clinical use. Gynecol Endocrinol 32:690–695. https://doi.org/10.1080/09513590.2016.1188282
Goud PT, Goud AP, Van Oostveldt P, Dhont M (1999) Presence and dynamic redistribution of type I inositol 1,4,5-trisphosphate receptors in human oocytes and embryos during in-vitro maturation, fertilization and early cleavage divisions. Mol Hum Reprod 5:441–451. https://doi.org/10.1093/molehr/5.5.441
Bizzarri M, Cucina A, Dinicola S, Harrath AH, Alwasel SH, Unfer V, Bevilacqua A (2016) Does myo-inositol effect on PCOS follicles involve cytoskeleton regulation? Med Hypotheses 91:1–5. https://doi.org/10.1016/j.mehy.2016.03.014
Nordio M, Basciani S, Camajani E (2019) The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: comparison with other ratios. Eur Rev Med Pharmacol Sci 23:5512–5521. https://doi.org/10.26355/eurrev_201906_18223
Unfer V, Carlomagno G, Papaleo E, Vailati S, Candiani M, Baillargeon JP (2014) Hyperinsulinemia alters myoinositol to d-chiroinositol ratio in the follicular fluid of patients with PCOS. Reprod Sci 21:854–858. https://doi.org/10.1177/1933719113518985
Acknowledgement
We are thankful to the Deanship of Scientific Research at Umm Al-Qura University for the financial support of our project (Project code 19-med-1-01-0043).
Author information
Authors and Affiliations
Contributions
WHA contributed to the study conception and design. The author has read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no interest dispute.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almalki, W.H. A review on inositol’s potential in cyclic disturbances of adipose-endocrinology-associated polycystic ovary syndrome. Mol Cell Biochem 476, 2943–2949 (2021). https://doi.org/10.1007/s11010-021-04123-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04123-3