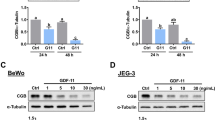
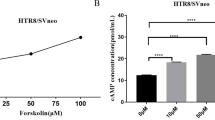
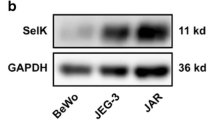
Abstract
Choriocarcinoma, a trophoblastic neoplasia, occurs in women as an incidence of abnormal pregnancy. BeWo choriocarcinoma cells derived from the abnormal placentation are a suitable model system to study the factors associated with differentiation, invasion and other cellular events as an alternative to clinical samples. Many protein kinases orchestrate the complex events of cell cycle and in case of malignancy such regulators are found to be mutated. In the present study, BeWo cells treated with forskolin (Fo) and phorbol 12-myristate 13-acetate (PMA) were used to study the role of PKA (protein kinase A) and PKC (protein kinase C), respectively, on the expression pattern of differentiation-related genes, membrane markers, PKC isoforms and cell cycle regulators. The effect of Fo and PMA on the cell proliferation was assessed. Progressive induction of alkaline phosphatase level and formation of multinucleated differentiated cells were observed in the cells treated with Fo. Exposure of cells to Fo and PMA induced the mRNA transcripts of α-hCG, β-hCG and endoglin and down-regulates E-cadherin at mRNA and protein levels. Synergistic levels of both up- and down-regulated genes/proteins were observed when cells were treated with the combination of Fo and PMA. The mRNA levels of cyclin D1, cyclin E1, p21, Rb, p53, caspase-3 and caspase-8 decreased gradually during differentiation. Fo significantly inhibited the protein levels of PCNA, Rb, PKC-α and PMA stimulated mRNA expression of PKC-ε and PKC-δ. Further, failure in the activation of essential components of the cell cycle machinery caused G2/M phase arrest in differentiating BeWo cells.










Similar content being viewed by others
References
Smith HO, Qualls CR, Prairie BA, Padilla LA, Rayburn WF, Key CR (2003) Trends in gestational choriocarcinoma: a 27-year perspective. Obstet Gynecol 102:978–987
Altieri A, Franceschi S, Ferlay J, Smith J, La Vecchia C (2003) Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol 4:670–678
Nelson DM (2015) How the placenta affects your life, from womb to tomb. Am J Obstet Gynecol 213:S12–S13. https://doi.org/10.1016/j.ajog.2015.08.015
Balagopal PG, Pandey M, Chandramohan K, Somanathan T, Kumar A (2003) Unusual presentation of choriocarcinoma. World J Surg Oncol 1:4–4. https://doi.org/10.1186/1477-7819-1-4
Stevens FT, Katzorke N, Tempfer C, Kreimer U, Bizjak GI, Fleisch MC, Fehm TN (2015) Gestational trophoblastic disorders: an update in 2015. Geburtshilfe Frauenheilkund 75:1043–1050. https://doi.org/10.1055/s-0035-1558054
Berkowitz RS, Goldstein DP (2009) Current management of gestational trophoblastic diseases. Gynecol Oncol 112:654–662. https://doi.org/10.1016/j.ygyno.2008.09.005
Yu P, Diao W, Jiang X (2016) A successfully treated metastatic choriocarcinoma coexistent with pregnancy: a case report of a 4-year follow-up. Medicine 95:e3505. https://doi.org/10.1097/MD.0000000000003505
Yaginuma Y, Yamashita T, Takuma N, Katayama H, Ishikawa M (1995) Analysis of the p53 gene in human choriocarcinoma cell lines. Br J Cancer 71:9–12
Kobayashi Y, Banno K, Shimizu T, Ueki A, Tsuji K, Masuda K, Kisu I, Nomura H, Tominaga E, Nagano O, Saya H, Aoki D (2013) Gene expression profile of a newly established choriocarcinoma cell line, iC3-1, compared to existing choriocarcinoma cell lines and normal placenta. Placenta 34:110–118. https://doi.org/10.1016/j.placenta.2012.11.003
Kaufmann P, Black S, Huppertz B (2003) Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69:1–7. https://doi.org/10.1095/biolreprod.102.014977
Bamberger AM, Bamberger CM, Aupers S, Milde-Langosch K, Loning T, Makrigiannakis A (2004) Expression pattern of the activating protein-1 family of transcription factors in the human placenta. Mol Hum Reprod 10:223–228. https://doi.org/10.1093/molehr/gah011
Neelima PS, Rao AJ (2008) Gene expression profiling during Forskolin induced differentiation of BeWo cells by differential display RT-PCR. Mol Cell Endocrinol 281:37–46. https://doi.org/10.1016/j.mce.2007.10.002
Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3rd (1986) Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118:1567–1582. https://doi.org/10.1210/endo-118-4-1567
Letamendia A, Lastres P, Almendro N, Raab U, Buhring HJ, Kumar S, Bernabeu C (1998) Endoglin, a component of the TGF-beta receptor system, is a differentiation marker of human choriocarcinoma cells. Int J Cancer 76:541–546
Webb PD, Todd J (1988) Attachment of human placental-type alkaline phosphatase via phosphatidylinositol to syncytiotrophoblast and tumour cell plasma membranes. Eur J Biochem 172:647–652
Coutifaris C, Kao LC, Sehdev HM, Chin U, Babalola GO, Blaschuk OW, Strauss JF 3rd (1991) E-cadherin expression during the differentiation of human trophoblasts. Development 113:767–777
Chen YX, Allars M, Maiti K, Angeli GL, Abou-Seif C, Smith R, Nicholson RC (2011) Factors affecting cytotrophoblast cell viability and differentiation: evidence of a link between syncytialisation and apoptosis. Int J Biochem Cell Biol 43:821–828. https://doi.org/10.1016/j.biocel.2011.02.007
Knerr I, Schubert SW, Wich C, Amann K, Aigner T, Vogler T, Jung R, Dotsch J, Rascher W, Hashemolhosseini S (2005) Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett 579:3991–3998. https://doi.org/10.1016/j.febslet.2005.06.029
Ji L, Brkic J, Liu M, Fu G, Peng C, Wang YL (2013) Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol Asp Med 34:981–1023. https://doi.org/10.1016/j.mam.2012.12.008
Lapenna S, Giordano A (2009) Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov 8:547–566. https://doi.org/10.1038/nrd2907
Bononi A, Agnoletto C, De Marchi E, Marchi S, Patergnani S, Bonora M, Giorgi C, Missiroli S, Poletti F, Rimessi A, Pinton P (2011) Protein kinases and phosphatases in the control of cell fate. Enzyme Res. https://doi.org/10.4061/2011/329098
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Nurse P (2000) A long twentieth century of the cell cycle and beyond. Cell 100:71–78
Wice B, Menton D, Geuze H, Schwartz AL (1990) Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res 186:306–316
Gottesman MM, Fleischmann RD (1986) The role of cAMP in regulating tumour cell growth. Cancer Surv 5:291–308
Mukherjee A, Park-Sarge OK, Mayo KE (1996) Gonadotropins induce rapid phosphorylation of the 3′,5′-cyclic adenosine monophosphate response element binding protein in ovarian granulosa cells. Endocrinology 137:3234–3245
Stork PJ, Schmitt JM (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266
Richards JS (2001) New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218
Sevetson BR, Kong X, Lawrence JC Jr (1993) Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci U S A 90:10305–10309
D’Angelo G, Lee H, Weiner RI (1997) cAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking Raf activation. J Cell Biochem 67:353–366
Chen J, Iyengar R (1994) Suppression of Ras-induced transformation of NIH 3T3 cells by activated G alpha s. Science 263:1278–1281
Thoresen GH, Johasen EJ, Christoffersen T (1999) Effects of cAMP on ERK mitogen-activated protein kinase activity in hepatocytes do not parallel the bidirectional regulation of DNA synthesis. Cell Biol Int 23:13–20. https://doi.org/10.1006/cbir.1998.0314
Steinberg SF (2008) Structural basis of protein kinase C isoform function. Physiol Rev 88:1341–1378
Parekh DB, Ziegler W, Parker PJ (2000) Multiple pathways control protein kinase C phosphorylation. EMBO J 19:496–503. https://doi.org/10.1093/emboj/19.4.496
Kharbanda S, Saleem A, Emoto Y, Stone R, Rapp U, Kufe D (1994) Activation of Raf-1 and mitogen-activated protein kinases during monocytic differentiation of human myeloid leukemia cells. J Biol Chem 269:872–878
He H, Wang X, Gorospe M, Holbrook NJ, Trush MA (1999) Phorbol ester-induced mononuclear cell differentiation is blocked by the mitogen-activated protein kinase kinase (MEK) inhibitor PD98059. Cell Growth Differ 10:307–315
Leszczyniecka M, Roberts T, Dent P, Grant S, Fisher PB (2001) Differentiation therapy of human cancer: basic science and clinical applications. Pharmacol Ther 90:105–156
Frey MR, Saxon ML, Zhao X, Rollins A, Evans SS, Black JD (1997) Protein kinase C isozyme-mediated cell cycle arrest involves induction of p21(waf1/cip1) and p27(kip1) and hypophosphorylation of the retinoblastoma protein in intestinal epithelial cells. J Biol Chem 272:9424–9435
Nakagawa M, Oliva JL, Kothapalli D, Fournier A, Assoian RK, Kazanietz MG (2005) Phorbol ester-induced G1 phase arrest selectively mediated by protein kinase C delta-dependent induction of p21. J Biol Chem 280:33926–33934. https://doi.org/10.1074/jbc.M505748200
Black JD (2000) Protein kinase C-mediated regulation of the cell cycle. Front Biosci 5:D406–D423. https://doi.org/10.2741/A522
Suzuki T, Ino K, Kikkawa F, Uehara C, Kajiyama H, Shibata K, Mizutani S (2002) Neutral endopeptidase/CD10 expression during phorbol ester-induced differentiation of choriocarcinoma cells through the protein kinase C- and extracellular signal-regulated kinase-dependent signalling pathway. Placenta 23:475–482. https://doi.org/10.1053/plac.2002.0820
Andersen B, Milsted A, Kennedy G, Nilson JH (1988) Cyclic AMP and phorbol esters interact synergistically to regulate expression of the chorionic gonadotropin genes. J Biol Chem 263:15578–15583
Orendi K, Gauster M, Moser G, Meiri H, Huppertz B (2010) The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction 140:759–766. https://doi.org/10.1530/REP-10-0221
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Garcia-Rozas C, Plaza A, Diaz-Espada F, Kreisler M, Martinez-Alonso C (1982) Alkaline phosphatase activity as a membrane marker for activated B cells. J Immunol 129:52–55
Sharma SC, Clemens JW, Pisarska MD, Richards JS (1999) Expression and function of estrogen receptor subtypes in granulosa cells: regulation by estradiol and forskolin. Endocrinology 140:4320–4334
Patil RH, Babu RL, Naveen Kumar M, Kiran Kumar KM, Hegde SM, Ramesh GT, Chidananda Sharma S (2015) Apigenin inhibits PMA-induced expression of pro-inflammatory cytokines and AP-1 factors in A549 cells. Mol Cell Biochem 403:95–106. https://doi.org/10.1007/s11010-015-2340-3
Boulaiz H, Prados J, Melguizo C, Garcia AM, Marchal JA, Ramos JL, Carrillo E, Velez C, Aranega A (2003) Inhibition of growth and induction of apoptosis in human breast cancer by transfection of gef gene. Br J Cancer 89:192–198. https://doi.org/10.1038/sj.bjc.6601064
Miller MJ, Foy KC, Kaumaya PT (2013) Cancer immunotherapy: present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov Med 15:166–176
Nampoothiri LP, Neelima PS, Rao AJ (2007) Proteomic profiling of forskolin-induced differentiated BeWo cells: an in-vitro model of cytotrophoblast differentiation. Reprod Biomed 14:477–487
Al-Nasiry S, Spitz B, Hanssens M, Luyten C, Pijnenborg R (2006) Differential effects of inducers of syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells. Hum Reprod 21:193–201. https://doi.org/10.1093/humrep/dei272
Huang R, Zhao L, Chen H, Yin RH, Li CY, Zhan YQ, Zhang JH, Ge CH, Yu M, Yang XM (2014) Megakaryocytic differentiation of K562 cells induced by PMA reduced the activity of respiratory chain complex IV. PLoS ONE 9:e96246. https://doi.org/10.1371/journal.pone.0096246
Pierce JG, Parsons TF (1981) Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495. https://doi.org/10.1146/annurev.bi.50.070181.002341
Sharma SC, Rao AJ (1995) Secretion is a stimulus for the synthesis of human chorionic gonadotrophin by first trimester human placenta. Biochem Mol Biol Int 36:1235–1241
Leisser C, Saleh L, Haider S, Husslein H, Sonderegger S, Knofler M (2006) Tumour necrosis factor-alpha impairs chorionic gonadotrophin beta-subunit expression and cell fusion of human villous cytotrophoblast. Mol Hum Reprod 12:601–609. https://doi.org/10.1093/molehr/gal066
Malhotra SS, Suman P, Gupta SK (2015) Alpha or beta human chorionic gonadotropin knockdown decrease BeWo cell fusion by down-regulating PKA and CREB activation. Sci Rep 5:11210. https://doi.org/10.1038/srep11210
Gougos A, St Jacques S, Greaves A, O’Connell PJ, d’Apice AJ, Buhring HJ, Bernabeu C, van Mourik JA, Letarte M (1992) Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int Immunol 4:83–92
Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M (1997) Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology 138:4977–4988. https://doi.org/10.1210/endo.138.11.5475
Kvanta A, Fredholm BB (1993) Synergistic effects between protein kinase C and cAMP on activator protein-1 activity and differentiation of PC-12 pheochromocytoma cells. J Mol Neurosci 4:205–214. https://doi.org/10.1007/bf02821552
Omata W, Ackerman WE, Vandre DD, Robinson JM (2013) Trophoblast cell fusion and differentiation are mediated by both the protein kinase C and a pathways. PLoS ONE 8:e81003. https://doi.org/10.1371/journal.pone.0081003
Uehara C, Ino K, Suzuki T, Kajiyama H, Kikkawa F, Nagasaka T, Mizutani S (2001) Upregulation of neutral endopeptidase expression and enzymatic activity during the differentiation of human choriocarcinoma cells. Placenta 22:540–549. https://doi.org/10.1053/plac.2001.0694
Rama S, Petrusz P, Rao AJ (2004) Hormonal regulation of human trophoblast differentiation: a possible role for 17beta-estradiol and GnRH. Mol Cell Endocrinol 218:79–94. https://doi.org/10.1016/j.mce.2003.12.016
Kale A, Soylemez F, Ensari A (2001) Expressions of proliferation markers (Ki-67, proliferating cell nuclear antigen, and silver-staining nucleolar organizer regions) and of p53 tumor protein in gestational trophoblastic disease. Am J Obstet Gynecol 184:567–574. https://doi.org/10.1067/mob.2001.111243
Babu RL, Naveen Kumar M, Patil RH, Devaraju KS, Ramesh GT, Sharma SC (2013) Effect of estrogen and tamoxifen on the expression pattern of AP-1 factors in MCF-7 cells: role of c-Jun, c-Fos, and Fra-1 in cell cycle regulation. Mol Cell Biochem 380:143–151. https://doi.org/10.1007/s11010-013-1667-x
Wei B, Xu C, Rote N (2012) Increased resistance to apoptosis during differentiation and syncytialization of BeWo choriocarcinoma cells. Adv Biosci Biotechnol 3:805–813. https://doi.org/10.4236/abb.2012.326100
Acknowledgements
The authors wish to express their gratitude to the Department of Science and Technology-PURSE [SR/59/Z-23/2010/38(c)] and University Grants Commission-CPEPA [8-2/2008(NS/PE)], Govt. of India for providing financial support. They also wish to express their gratitude to the Department of Microbiology and Biotechnology, Bangalore University, Bengaluru for providing the DST-FIST, UGC-SAP and laboratory facility. They wish to thank Prof. P. Kondaiah, Dept. of MRDG, IISc, Bengaluru for providing the flow cytometry facility. The author Naveen Kumar M., thank Prof. M. V. V. Subramanyam and Late E. Vijayalakshmi for their support during his research and greatly acknowledges the Council for Scientific and Industrial Research (CSIR), Govt. of India for the award of Senior Research Fellowship (09/039 (0116)/2016 EMR-I).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Naveen Kumar, M., Babu, R.L., Patil, R.H. et al. Protein kinases orchestrate cell cycle regulators in differentiating BeWo choriocarcinoma cells. Mol Cell Biochem 452, 1–15 (2019). https://doi.org/10.1007/s11010-018-3407-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3407-8