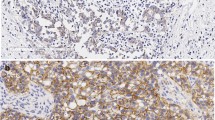
Abstract
The identification of informative biomarkers that could predict the treatment response is particularly important in the triple-negative (TN) breast cancer, which is characterized by biological diversity. The aim of this study was to investigate the impact of vascular endothelial growth factor receptor (VEGFR2) expression and its gene polymorphisms on pathologic complete response (pCR) to neoadjuvant chemotherapy (NCT) in Russian patients with TN breast cancer. We performed a retrospective analysis of 70 women with operable TN breast cancer, who underwent NCT with 5-fluorouracil, adriamycin, and cyclophosphamide (FAC) or cyclophosphamide, adriamycin, and capecitabine (CAX) between 2007 and 2013. VEGFR2 expression was evaluated before NCT by immunohistochemistry. TaqMan SNP assays were used for genotyping KDR − 604T>C (rs2071559) and KDR 1192G>A (rs2305948) polymorphisms. The pCR was used as an end-point in the treatment efficacy analysis. In the univariate analysis, the pCR rate was strongly associated with young age (P = 0.004), high Ki67 expression (P = 0.012), lymph node negativity (P = 0.023) as well as with positive VEGFR2 expression (P = 0.019) and the CAX regimen (P = 0.005). In the multivariate analysis, only patient’s age (P = 0.005) and pre-NCT VEGFR2 expression (P = 0.048) remained significant predictors of pCR. The pCR rate was higher in the CAX-treated patients than that in the FAC-treated patients (P = 0.005). Our results revealed that − 604TT genotype of rs2071559 and age < 50 years were correlated with a pCR in the CAX-treated patients. VEGFR2 expression in pre-NCT tumors and KDR gene polymorphism can be considered as additional predictive molecular markers of pCR in Russian TN breast cancer patients treated with NCT.

Similar content being viewed by others
References
Tomao F, Papa A, Zaccarelli E, Rossi L, Caruso D, Minozzi M, Vici P, Frati L, Tomao S (2015) Triple-negative breast cancer: new perspectives for targeted therapies. Onco Targets Ther 8:177–193. https://doi.org/10.2147/OTT.S67673
Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A (2010) Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev 36(3):206–215. https://doi.org/10.1016/j.ctrv.2009.12.002
Kuligina ES (2010) Epidemiological and molecular aspects of breast cancer. Pract Oncol 11(4):203–216
Choinzonov EL, Pisareva LF, Odintsova IN, Ananina OA, Boyarkina AP (2014) The state of oncological service in Siberia and Far East. Health Care Russ Fed 58(3):10–14
Gnant M, Thomssen C, Harbeck N (2015) St. Gallen/Vienna 2015: a brief summary of the consensus discussion. Breast Care (Basel) 10(2):124–130. https://doi.org/10.1159/000430488
Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM (2013) Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 18(2):123–133. https://doi.org/10.1634/theoncologist.2012-0397
Dales JP, Garcia S, Carpentier S, Andrac L, Ramuz O, Lavaut MN, Allasia C, Bonnier P, Taranger-Charpin C (2004) Prediction of metastasis risk (11 year follow-up) using VEGF-R1, VEGF-R2, Tie-2/Tek and CD105 expression in breast cancer (n = 905). Br J Cancer 90(6):1216–1221
Ghosh S, Sullivan CA, Zerkowski MP, Molinaro AM, Rimm DL, Camp RL, Chung GG (2008) High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol 39(12):1835–1843. https://doi.org/10.1016/j.humpath.2008.06.004
Dhakal HP, Naume B, Synnestvedt M, Borgen E, Kaaresen R, Schlichting E, Wiedswang G, Bassarova A, Holm R, Giercksky KE, Nesland JM (2012) Expression of vascular endothelial growth factor and vascular endothelial growth factor receptors 1 and 2 in invasive breast carcinoma: prognostic significance and relationship with markers for aggressiveness. Histopathology 61(3):350–364. https://doi.org/10.1111/j.1365-2559.2012.04223.x
Jansson S, Bendahl PO, Grabau DA, Falck AK, Fernö M, Aaltonen K, Rydén L (2014) The three receptor tyrosine kinases c-KIT, VEGFR2 and PDGFRα, closely spaced at 4q12, show increased protein expression in triple-negative breast cancer. PLoS ONE 9(7):e102176. https://doi.org/10.1371/journal.pone.0102176
Yan JD, Liu Y, Zhang ZY, Liu GY, Xu JH, Liu LY, Hu YM (2015) Expression and prognostic significance of VEGFR-2 in breast cancer. Pathol Res Pract 211(7):539–543. https://doi.org/10.1016/j.prp.2015.04.003
Wang Y, Zheng Y, Zhang W, Yu H, Lou K, Zhang Y, Qin Q, Zhao B, Yang Y, Hui R (2007) Polymorphisms of KDR gene are associated with coronary heart disease. J Am Coll Cardiol 50(8):760–767
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Babyshkina N, Vtorushin S, Zavyalova M, Patalyak S, Dronova T, Litviakov N, Slonimskaya E, Kzhyshkowska J, Cherdyntseva N, Choynzonov E (2017) The distribution pattern of ERα expression, ESR1 genetic variation and expression of growth factor receptors: association with breast cancer prognosis in Russian patients treated with adjuvant tamoxifen. Clin Exp Med 17(3):383–393. https://doi.org/10.1007/s10238-016-0428-z
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. https://doi.org/10.1200/JCO.2009.25.6529
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel Members (2015) Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 26(8):1533–1546. https://doi.org/10.1093/annonc/mdv221
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10(16):5367–5374. https://doi.org/10.1158/1078-0432.CCR-04-0220
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Guarneri V, Piacentini F, Ficarra G, Frassoldati A, D’Amico R, Giovannelli S, Maiorana A, Jovic G, Conte P (2009) A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol 20(7):1193–1198. https://doi.org/10.1093/annonc/mdn761
Makhoul I, Griffin RJ, Siegel E, Lee J, Dhakal I, Raj V, Jamshidi-Parsian A, Klimberg S, Hutchins LF, Kadlubar S (2016) High- circulating Tie2 is associated with pathologic complete response to chemotherapy and antiangiogenic therapy in breast cancer. Am J Clin Oncol 39(3):248–254. https://doi.org/10.1097/COC.0000000000000046
Babyshkina N, Malinovskaya E, Patalyak S, Bragina O, Tarabanovskaya N, Doroshenko A, Slonimskaya E, Perelmuter V, Cherdyntseva N (2014) Neoadjuvant chemotherapy for different molecular breast cancer subtypes: a retrospective study in Russian population. Med Oncol 31(9):165. https://doi.org/10.1007/s12032-014-0165-7
Allegrini G, Coltelli L, Orlandi P, Fontana A, Camerini A, Ferro A, Cazzaniga M, Casadei V, Lucchesi S, Bona E, Di Lieto M, Pazzagli I, Villa F, Amoroso D, Scalese M, Arrighi G, Molinaro S, Fioravanti A, Finale C, Triolo R, Di Desidero T, Donati S, Marcucci L, Goletti O, Del Re M, Salvadori B, Ferrarini I, Danesi R, Falcone A, Bocci G (2014) Pharmacogenetic interaction analysis of VEGFR-2 and IL-8 polymorphisms in advanced breast cancer patients treated with paclitaxel and bevacizumab. Pharmacogenomics 15(16):1985–1999. https://doi.org/10.2217/pgs.14.140
Acknowledgements
The study was supported by the ERA Net RUS Plus S&T CHIT-ALPHA-THER grant, the Russian Foundation for Basic Research (Project No. 16-54-76015). We acknowledge support of this work by the Tomsk State University Competitiveness Improvement Program. We are grateful to Dr. Viktoriya Stalbovskaya and Dr. Matvey Tsyganov for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Babyshkina, N., Zavyalova, M., Tarabanovskaya, N. et al. Predictive value of vascular endothelial growth factor receptor type 2 in triple-negative breast cancer patients treated with neoadjuvant chemotherapy. Mol Cell Biochem 444, 197–206 (2018). https://doi.org/10.1007/s11010-017-3244-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3244-1