Abstract
Dietary measures and plant–based therapies as prescribed by native systems of medicine have gained attraction among diabetics with claims of efficacy. The present study investigated the effects of S-Allylcysteine (SAC) on body weight gain, glucose, insulin, insulin resistance, and nitric oxide synthase in plasma and argininosuccinate synthase (AS) and argininosuccinate lyase (ASL), lipid peroxides and antioxidant enzymes in aorta of control and streptozotocin-nicotinamide (STZ-NA)-induced diabetic rats. Changes in body weight, glucose, insulin, insulin resistance, and antioxidant profiles of aorta and mRNA expressions of nitric oxide synthase, AS, and ASL were observed in experimental rats. SAC (150 mg/kg b.w) showed its therapeutic effects similar to gliclazide in decreasing glucose, insulin resistance, lipid peroxidation, and increasing body weight; insulin, antioxidant enzymes, and mRNA levels of nitric oxide synthase, argininosuccinate synthase, and argininosuccinate lyase genes in STZ-NA rats. Histopathologic studies also revealed the protective nature of SAC on aorta. In conclusion, garlic and its constituents mediate the anti-diabetic potential through mitigating hyperglycemic status, changing insulin resistance by alleviating endothelial dysregulation in both plasma and tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is attaining a level of pandemic significance all over the world, and the circumstances in developing countries are worsening day by day [1]. DM is undoubtedly perceived as a noteworthy danger to human wellbeing, and the high predominance of mortality among diabetic patients is due to the cardiovascular complications that are involved in DM. The main prevalent form of diabetes is type 2 (T2DM), which accounts for about 90% of all cases of DM [2, 3]. Data depict that more than 75% of all hospitalizations in diabetic subjects are attributed to the cardiovascular complications [4] that affect many tissues, including microvasculature, macrovasculature, nerve, and the heart [5].
Endothelial dysfunction, the first step in atherosclerosis, is interconnected with hypertension, diabetes, and chronic heart failure [6]. Vascular tone and homeostasis are regulated by endothelium by generating a number of autacoids. Nitric oxide (NO) is one of the vasoactive autacoids, regulating vascular tone and homeostasis, and it is originally identified as endothelium-derived relaxing factor [7]. In diabetic patients, endothelial dysfunction gives off an impression of being a predictable discovery; indeed, there is general accordance in the fact that hyperglycemia and diabetes lead to an obstruction in NO production and activity [8]. The inability of endothelial cells to release NO in response to physiological cues that promote vasodilatation is the hallmark of endothelial dysfunction [9]. Endothelial nitric oxide synthase (eNOS), arginino succinate synthase (AS), and argininosuccinate lyase (ASL) are core components for endothelial NO production [10]. The conversion of citrulline to arginine is the principal role of AS and AL. AS is rate-limiting factor to the citrulline–NO cycle and as such is required to sustain endothelial function and viability [11].
There has been an increased interest in oxidative stress and its role in the development of complications of diabetes in the recent decade [12]. Overloaded superoxide can react with NO, forming the toxic peroxynitrite, which in turn uncouples nitric oxide synthase (eNOS) by oxidizing the essential NOS redox-sensitive cofactor tetrahydrobiopterin and causes eNOS to produce more superoxide anion [6]. Extenuating the highly prevalent plaque T2DM is of high precedence in both the developed and developing countries, and the scientific mission on agents ameliorating the cardio-metabolic risk is increasing in the recent past. Many synthetic drugs are available for the treatment of diabetes. However, the use of conventional medicines results in side effects. Recently, many researchers are seeking natural products or dietary interventions to prevent or treat T2DM. Apart from the traditional anti-diabetic treatment, antioxidant therapy may benefit in diabetes. It is understood that the positive role of antioxidant activity will achieve good outcomes in T2DM and its long-term complications [13].
The potent therapeutic limit of garlic and its components has been reported by many researchers utilizing multiple in vivo frameworks [14, 15]. S-Allylcysteine (SAC), a derivative of garlic, is a sulfur-containing amino acid [16]. Previously, it was reported that SAC exhibits insulin-like antihyperglycemic effect in the STZ-induced diabetic rats [17] and also reverses the changes in the levels of glucose metabolism in liver [18]. Although the anti-hyperglycemic and antioxidant activities of SAC have been widely studied, there are no reports pertaining to the hyperglycemia-induced oxidative stress and improving endothelial dysfunction in diabetic animals. Therefore, this study aims to evaluate the effect of SAC in narrowing hyperglycemia-induced ROS formation and improving endothelial dysfunction in aorta of streptozotocin (STZ)-nicotinamide (NA)-induced diabetic rats.
Materials and methods
Animals
Male Wistar rats (Rattus norvegicus) weighing about 150–180 g were used as experimental animals in the present investigation. The animals were maintained in the central animal facility room, Muthayammal College of Arts and Science, Rasipuram, Tamilnadu, India and were fed a standard pellet diet (AMRUT, PUNE, INDIA) and water ad libitum. The protocol of this study was approved by the institutional ethical committee of College of Arts and Science, Rasipuram, Tamilnadu, India (1416/P0/a/11/CPCSEA).
Chemicals
SAC (99%) was commercially available, and it was purchased from LGC Prochem, Bangalore, India. Streptozotocin was purchased from Himedia, Bangalore, India. All other reagents used in the experiments were of analytic grade and of the highest purity.
Induction of diabetes
The overnight-fasted rats were made diabetic by a single intraperitoneal injection of freshly prepared STZ (55 mg/kg body weight) in citrate buffer (0.1 M, pH 4.5) in a volume of 1 ml/kg 15 min after the intraperitoneal administration of nicotinamide (110 mg/kg b.w). Hyperglycemia was confirmed by the elevated glucose levels (Above 250 mg/dl) in blood, determined at 72 h and then on day 7 after injection.
Experimental design
After the successful induction of experimental diabetes, the rats were divided into four groups each comprising a minimum of six rats.
Group 1: Control rats.
Group 2: Diabetic control rats.
Group 3: STZ-NA-treated rats were given SAC (150 mg/kg body weight) in vehicle solution orally for 45 days using an intragastric tube [18].
Group 4: STZ-NA-treated rats were given gliclazide (5 mg/kg b.w/rat) in vehicle solution orally for 45 days using an intragastric tube [19].
Body weight and blood glucose level measurements were conducted periodically. At the end of the experiment, blood was collected from overnight-fasted animals under inhalation of anesthesia by retro orbital puncture method. Blood samples were collected into tubes containing with or without anticoagulant. The samples were centrifuged at 250×g for 5 min at 4 °C, and then the plasma was immediately removed and stored at −20 °C until further analysis. Serum NO level was assayed by the Griess method [20]. A plasma level of insulin was determined using kits from bio-Merieux, RCS, Lyon, France. Insulin resistance was calculated using the homeostasis model assessment. NO synthase activity was estimated using the Kit from Calbiochem, USA (Catalogue Number 482702). AS and ASL activities were estimated by the modified method of Levin (1971) as described by Swamy et al. [21].
Aorta tissues were quickly excised and placed in a petri dish, washed in ice-cold saline, and weighed. The tissues were homogenized in 0.25 M sucrose, 0.02 M triethanolamine hydrochloride buffer at pH 7.4, then centrifuged at 1000 g for 10 min, and finally, the supernatant was collected. The supernatant was used for the estimation of thiobarbutric acid-reactive substance (TBARS), hydroperoxide, GSH, SOD, CAT, and GPx. All these parameters were analyzed using commercially available kits (Sigma Aldrich, USA).
RT-PCR analysis
Total RNA was isolated from the aorta tissue by using tri-reagent (Sigma–Aldrich, USA) according to manufacturer’s protocol and reverse transcribed to obtain cDNA using DNA synthesis kit (Applied Bio systems, Foster City, USA). 20 ng of cDNA was used for semi-quantitative polymerase chain reaction (qPCR). The PCR amplification was performed for 38 cycles using the following cycling conditions: 30 s of denaturation at 94 °C, 30 s of annealing at 59 °C, and 1 min of extension at 72 °C, with the primers. The sequences of the primers are given in Table 1. The housekeeping gene β actin was used for normalization.
Histopathological studies
Harvested aortic tissues from the sacrificed animals were fixed in 10% neutral buffered formalin solution, dehydrated in ethanol, and embedded in paraffin. Sections of 5 µm thickness were prepared using a rotary microtome and stained with hematoxylin and eosin dye for microscopic observations.
Statistical analysis
All the results were expressed as the Mean ± S.D. for six animals in each group. All the grouped data were statistically evaluated using SPSS\10.0 software. Hypothesis testing methods included one way analysis of variance (ANOVA) followed by least significant difference test; significance level at p < 0.05 was considered to indicate statistical significance.
Results
Table 2 indicated the level of plasma glucose, plasma insulin, insulin resistance, and body weight development in control and STZ-NA-induced experimental diabetic rats. Significant reductions in the levels of insulin and bodyweight and concomitant increases in the levels of plasma glucose and insulin resistance were observed in STZ-NA diabetic rats and these levels were normalized after treatment with SAC and gliclazide.
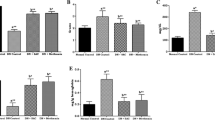
Figure 1a–c shows activities of NO, AS, ASL, and the mRNA expressions of normal and experimental rats. There were significant declines in the activities of (p < 0.05) in NO, AS, and ASL. Oral treatment with SAC or gliclazide for 45 days to STZ-NA rats significantly increased the activities of NO, AS, and ASL. The effects of SAC treatment on NO, AS, and ASL mRNA levels in the tissue were examined by RT-PCR. The levels of NO, AS, and ASL mRNA were found to decrease in the STZ-NA control rats. However, treatment with SAC or gliclazide resulted in significant increases in NO, AS, and ASL mRNA levels.
a Effect of SAC treatment on activities of NO, AS, and ASL of diabetic rats. Values are expressed as Mean ± SD for 6 animals in each group. Values are statistically significant at *p < 0.05. aSignificantly different from control. bSignificantly different from diabetic control. b Effect of SAC treatment on aortic NO, AS, and ASL mRNA levels. L1—control rats; L2—diabetic control rats; L3—diabetic rats treated with SAC; L4—diabetic rats treated with gliclazide. c Fold changes in mRNA levels of aortic NO, AS, and ASL. Values are expressed as Mean ± SD for 6 animals in each group. Values are statistically significant at *p < 0.05. aSignificantly different from control bSignificantly different from diabetic control
Figures 2 and 3 reveal the levels of TBARS, GSH, SOD, CAT, and GPx in the aortas of control and STZ-NA-induced experimental diabetic rats. Significant reductions in the levels of GSH, SOD, CAT, and GPx and a concomitant increase in the level of TBARS were observed in STZ - NA-induced experimental diabetic rats. Treatment with SAC or gliclazide showed significant increases in the activities of SOD, CAT, and GPx and a decrease in the level of TBARS in aorta of treated animals.
Effects of SAC on GSH, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in aortas of normal and experimental diabetic rats. Values are mean ± SD, n = 6. Values are statistically significant at *p < 0.05. aSignificantly different from normal control. bSignificantly different from diabetic control. U; 50% of inhibition of epinephrine auto oxidation per min for SOD; U; µmole of hydrogen peroxide decomposed per min per mg of protein for catalase; U; µmole of glutathione oxidized per min per mg of protein for GPx
The histopathological observations in control and STZ-NA-induced experimental diabetic rats are depicted in Fig. 4. The aorta of control rats (Fig. 4a) showed a normal architecture with regular aortic morphology. There was a massive fibrillar thickening with extensive proliferation of smooth muscle cells and degeneration of tunica intima and appearance of foam cells in diabetic control rats (Fig. 4b). Treatment of SAC to diabetic rats showed slight intimal thickening characterized by proliferation of smooth muscle cells (Fig. 4c). Treatment of gliclazide to diabetic rats showed a regular morphology of aortic intima (Fig. 4d).
Effect of SAC in aorta of experimental rats a Normal control: normal control group was shown to have normal architecture with regular aortic morphology; H&E X40. b Diabetic control: diabetic control histopathology showed massive fibrillar thickening with extensive proliferation of smooth muscle cells and degeneration of tunica intima and appearance of foam cells; H&E X40. c SAC administration showed slight intimal thickening characterized by proliferation of smooth muscle cells. d Gliclazide administration showed regular morphology of aortic intima; H&E X40
Discussion
In order to better comprehend the pathogenesis, hereditary variables and biological complications involved in T2DM, and to better investigate the different remedial agents, appropriate experimental models are important. Some are additionally known to have pathological syndromes similar to T2DM [22]. Administration of both STZ and NA has been projected to provoke investigational DM in the rat. STZ is well recognized to root pancreatic β-cell damage, while NA is administered to rats to incompletely guard insulin-secreting cells against STZ. STZ is transported into β-cells via the glucose transporter 2 and causes DNA damage which leads to enhanced activity of poly (ADP-ribose) polymerase-1 to repair DNA. Conversely, overstated activity of this enzyme results in diminution of intracellular NAD (+) and ATP, and the insulin secreting cells undergo necrosis. The protecting action of NA is due to the inhibition of polymerase-1 activity. NA inhibits this enzyme and put off the reduction of NAD (+) and ATP in cells exposed to STZ. The cruelty of diabetes in experimental rats powerfully depends on the doses of STZ and NA administered to these animals. Hence, in diabetic rats, blood glucose may be altered in a broad range from slight hyperglycemia to extensive hyperglycemia compared with control animals [23]. T2DM is the outcome of various deformities including insulin resistance of peripheral tissues to the glucose utilizing effect of insulin and augmented hepatic glucose production [24]. The most particular enviable avenues and remnant mechanism for the prevention and management of T2DM and cardiovascular diseases is dietary control which may enhance the endogenous antioxidant system [25].
Diabetes is portrayed by a severe loss in body weight, which might be the result of protein wasting because of inaccessibility of sugar as a vitality source [26]. In the present study, significant elevation in blood glucose level, insulin resistance, and decreased body weight gain, insulin was observed in experimental type-2 diabetic rats when compared to normal control rats. This is in contrast to diet-induced type-2 diabetic animals, in which increased blood glucose is accompanied by increased blood insulin and hyperglycemia results from insulin resistance [27]. In humans with type-2 diabetes, glucose challenge induces a rise in blood glucose which is markedly higher than in healthy people and is comparable with that observed in rats with STZ–NA-induced diabetes. However, in type-2 diabetic humans, hyperglycemia observed after glucose load usually results from both insulin resistance and impaired function of β–cells [28]. Hence, in addition to glycemic control, management of impaired function of β-cells is also essential for controlling insulin resistance and limiting the complications of non-insulin-dependent diabetes mellitus (NIDDM). Hyperglycemia in type-2 diabetes is there to a limited extent because of the absence of concealment of hepatic glucose production in the absorptive state and unwarranted glucose production in the post absorptive state. Catalysts that direct hepatic glucose metabolism are potential focuses for controlling hepatic glucose balance and subsequently blood glucose levels in T2DM. During diabetes, diminished activities of hexokinase and elevated activities of glucose-6-phosphatase and fructose-6- phosphatase are found which is due to the total absence or insufficiency of insulin [29]. SAC up directs the activities of both these enzymes in hepatic tissues through insulin discharge and thereby it enhances the utilization of glucose for cellular biosynthesis, which is marked by the significant decrease in plasma glucose levels. Earlier, we reported the insulin-like antihyperglycemic effect of SAC in the STZ-induced diabetic rats [19] and also reversed the changes in the levels of glucose metabolism in liver by altering the metabolic key enzymes [22].
T2DM is associated with severe cardiovascular complications due to which diabetic vascular disease gets much attention. High concentrations of glucose have been associated with endothelial dysfunction [30]. The decreased availability of arginine and impaired synthesis of NO is seen to be important in the development of endothelial dysfunction and also the decreased activity and/or expression of eNOS or increased degradation of NO secondary to enhanced superoxide production could be the mechanism underlying this endothelial dysfunction. During diabetes, endothelial NO production is impaired and it plays a pivotal role in the pathogenesis of diabetes, compromising endothelial cell regulation of vascular function and homeostasis [31]. Together, the present study confirms attenuation of hyperglycemia-induced oxidative stress and endothelial dysfunction by oral treatment of SAC by restoring the NO bioavailability. NO, a potent chemical mediator, is severely restricted not only by the downregulation of eNOS [32] but also by the availability of arginine provided by AS via the citrulline-NO cycle. Organized upregulation of eNOS expression has been identified previously in a number of systems [33]. In the present study, diminished levels of serum NO and expression of eNOS mRNA were observed in STZ-NA diabetic rats. This might be due to the limited availability of arginine which could lead to enzymatic uncoupling of eNOS with subsequent production of reactive oxygen species [34]. SAC therapy to the diabetic rats resulted in an increased expression of eNOS which could produce a large amount of NO.
Endothelial AS and ASL, the rate-limiting enzymes in the recycling of citrulline to arginine, are critical in sustaining NO production in endothelial cells [35]. It is well known that the post-translational regulation of AS and ASL would play a significant role in maintaining NO homeostasis [36]. It was reported that AS is prone to a regulatory mechanism by change in intracellular calcium level. Calcium-dependent activation of insulin resulted in an increase in phosphorylation AS at Ser-328 for eNOS activation. Calcium rich environment favors formation of soluble proinsulin [37]. A diminished expression of AS and ASL mRNA observed in this study might be due to the decreased level of insulin signaling. Oral administration of SAC to the diabetic rats increased the expressions of AS and ASL that might be due to enhanced insulin signaling which is required to maintain a functional citrulline–NO cycle [38].
Oxidative stress-induced hyperglycemia plays an important role in the development of diabetic complications [39] particularly vascular diseases involving both the macrovasculature and microvasculature. Aorta represents state of tissue macrovasculature in diabetic complications. Overproductions of oxidative free radicals or reactive oxygen species (ROS) are attributed to oxidative stress resulting in lipid peroxidation and subsequently increased malondialdehyde levels and other TBARS levels. In the present study, the TBARS and hydroperoxides levels in the STZ-NA control animals were higher than those for normal control animals. Increased levels of TBARS and hydroperoxides in STZ-NA control animals indicated enhanced lipid peroxidation leading to tissue damage and failure of the endogenous antioxidant defense mechanisms that prevent the overproduction of ROS. Oral treatment with SAC significantly reduced the levels of lipid peroxidation markers, which could be a result of improved glycemic control and antioxidants status. A previous study has provided the support that, due to the antioxidant function, SAC can reduce lipid peroxidation in cardiac membranes [40].
Glutathione (GSH), a metabolic regulator and putative indicator of health, is involved in the protection of normal cell structure and function, by maintaining the redox homeostasis, quenching of free radicals, and participating in detoxification reactions [41]. It is a direct scavenger of free radicals as well as a co-substrate for peroxide detoxification by glutathione peroxidases [42]. Depleted glutathione content was reported in experimental-induced diabetes [39]. The reduced GSH may be due to reduction in its synthesis or to its degradation by oxidative stress in diabetic animals [42]. SOD and CAT are involved in the clearance of superoxide and hydrogen peroxide radicals, respectively. GPx has been shown to be an important adaptive response to condition of increased peroxidative stress. In the present study, decreased aortic SOD, CAT, and GPx activities were found in STZ-NA control animals. During diabetes, excessive production of hydrogen peroxide due to the auto-oxidation of glucose, protein glycation, and lipid oxidation led to a marked decline in the enzymatic antioxidants. SAC treatment significantly increased the aortic glutathione content; and SOD, CAT, and GPx activities in experimental diabetic animals. Administration of SAC increased GSH level, and it may in turn activate the GSH-dependent enzymes such as glutathione peroxidase and glutathione-S-transferase. This might be due to the presence of hydroxyl group in SAC which could exert antioxidant and free radical properties [39].
In conclusion, the present study investigated the effects of SAC in narrowing hyperglycemia-induced ROS formation and improving endothelial dysfunction. It was concluded that oral treatment with SAC was able to modulate the vascular endothelial dysfunction by altering AS and ASL protein expressions and aortic antioxidant enzymes indicating that this compound can mitigate the hydroxyl-radical accumulation and consequently improve antioxidant defense system.
This research topic addressed the effect of SAC on type-2 diabetes and also stressed the need for the better treatment for type-2 diabetes. These results may contribute to better understanding of the anti-hyperglycemic role of SAC, emphasizing the influence of this antioxidant in the diet for human health, possibly by preventing cardiovascular disorders associated with diabetes mellitus.
This study adds that the consumption of vegetables and fruits is a major focus of dietary strategies for disease prevention. It can, therefore, be concluded that the SAC showed an astonishing anti-diabetic effect on rats. It is most likely because of high antioxidant nature of SAC which may exert these preventing effects. This study shall give the idea of having suitable herbal bioformulation having SAC as its one of the constituents.
References
Kumar A, Sharma SS (2010) NFKB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun 394:360–365
ADA—American Diabetes Association (2009), Diagnosis and classification of diabetes mellitus. Diabet Care 32:S62–S67
Rodbard HW, Jellinger PS, Davidson JA (2009) Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endoc Pract 15:540–559
Wang Y, Ying L, Chen YY (2014) Induction of heme oxygenase-1 ameliorates vascular dysfunction in streptozotocin-induced type 2 diabetic rats. Vasc Pharmacol 61:16–24
Thent ZC, Lin TS, Das S (2012) Effect of Piper sarmentosum extract on the cardiovascular system of diabetic sprague-dawley rats: electron microscopic study. Evid-Based Comp Alter Med 7:1–9
Schulz E, Jansen T, Wenzel P, Daiber A (2008) Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10:1115–1126
Hayashi T, Yano K, Matsui-Hirai H (2008) Nitric oxide and endothelial cellular senescence. Pharmacol Therap 120:333–339
Bakker W, Eringa EC, Sipkema P, Van Hinsbergh VW (2009) Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 335:165–189
Erez A, Nagamani SC, Shchelochkov OA (2011) Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 17:1619–1626
Husson A, Brasse-Lagnel C, Fairand A (2003) Argininosuccinate synthetase from the urea cycle to the citrulline–NO cycle. Eur J Biochem 270:1887–1899
Lau YS, Tian XY, Huang Y, Murugan D (2013) Boldine protects endothelial function in hyperglycemia-induced oxidative stress through an antioxidant mechanism. Biochem Pharmacol 85:367–375
Taye A, Wind S (2010) Role of NADPH oxidase in the endothelial dysfunction and oxidative stress in aorta of aged spontaneous hypertensive rats. Iran J Basic Med Sci 13:48–56
Adefegha SA, Oboh G, Adefegh O (2014) Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high-fat diet/streptozotocin-induced diabetes rat model. J Sci Food Agric 94:2726–2737
Hassana HA, Hafezb HS, Zeghebara FE (2010) Garlic oil as a modulating agent for oxidative stress and neurotoxicity induced by sodium nitrite in male albino rats. Food Chem Toxicol 48:1980–1985
Hfaiedha N, Muratb JC, Elfekia A (2011) Protective effects of garlic (Allium sativum) extract upon lindane-induced oxidative stress and related damages in testes and brain of male rats. Pest Biochem Physiol 100:187–192
Saravanan G, Ponmurugan P (2011) Ameliorative potential of S-allyl cysteine on oxidative stress in STZ induced diabetic rats. Chem Biol Interact 189:100–106
Brahmanaidu P, Uddandrao VVS, Pothani S, Naik RR, Begum MS, Varatharaju C, Pandiyan R, Saravanan G (2016) Effects of S-allylcysteine on biomarkers of polyol pathway in experimental type II diabetes in rats. Can J Diabetes 40:442–448
Saravanan G, Ponmurugan P, Senthil kumar GP, Rajarajan T (2009) Modulatory effect of S-allylcysteine on glucose metabolism in streptozotocin induced diabetic rats. J Funct Foods 1:336–340
Pulido N, Suarez A, Casanova B (1997) Glyclazide treatment of streptozotocin diabetic rats restores GLUT4 protein content and basal glucose uptake in skeletal muscle. Metabolism 46: 10–13
Wang SX, Xiong XM, Song T (2005) Protective effects of cariporide on endothelial dysfunction induced by high glucose. Acta Pharmacol Sin 26:329–333
Swamy M, Adlin ZZ, Chandran G (2005) Effect of acute ammonia toxicity on nitric oxide (NO), citrulline-NO cycle enzymes, arginase and related metabolites in different regions of rat brain. Neurosci Res 53:116–122
Pari L, Rajarajeswari N (2009) Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chem Biol Interact 181:292–296
Szkudelski T (2012) Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med 237(5):481–490
Oboh G, Akinyemi AJ, Ademiluyi AO, Adefegha SA (2010) Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. J Food Nutr Res 49:14–20
Pari L, Sankaranarayanan C (2009) Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin–nicotinamide induced diabetic rats. Life Sci 85:830–834
Lindstrom P (2010) The physiology of obese-hyperglycemic mice (ob/ob mice). Scient World J 7:666–685
Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes. J Clin Invest 116:1802–1812
Anwer T, Sharma M, Pillai KK, Iqbal M (2008) Effect of withania somnifera on insulin sensitivity in non-insulin-dependent diabetes mellitus rats. Basic Clini Pharmacol Toxicol 102:498–503
Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B (2014) Modulatory effects of diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in streptozotocin-induced diabetic rats. Can J Diabetes 38:409–414
Ting HH, Timimi FK, Boles KS, Creager SJ (1996) Vitamin C improves endothelium-dependent vasodilation in patients with noninsulin- dependent diabetes mellitus. J Clin Invest 97:22–28
Anderson HD, Rahmutula D, Gardner DG (2004) Tumor necrosis factor-α inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem 279:963–969
Oyadomari S, Gotoh T, Aoyagi K, Araki E (2001) Coinduction of endothelial nitric oxide synthase and arginine recycling enzymes in aorta of diabetic rats. Nitric Oxide 5:252–260
Goodwin BL, Solomonson LP, Eichler DC (2004) Argininosuccinate synthase expression is required to maintain nitric oxide production and cell viability in aortic endothelial cells. J Biol Chem 279:18353–18360
Haines RJ, Karen Corbin D, Laura Pendleton C (2012) Insulin transcriptionally regulates argininosuccinate synthase to maintain vascular endothelial function. Biochem Biophys Res 421:9–14
Dodson G, Steiner D (1998) The role of assembly in insulin’s biosynthesis. Curr Opin Struct Bio l8:189–194
Van Reyk DM, Gillies MC, Davie MJ (2003) The retina: oxidative stress and diabetes. Redox Rep 8:187–192
Padmanabhan M, Prince SM (2006) Preventive effect of S-allylcysteine on lipid peroxides and antioxidants in normal and isoproterenol-induced cardiotoxicity in rats: a histopathological study. Toxicology 224:128–137
Winterbourn CC (1995) Concerted antioxidant activity of glutathione and superoxide dismutase, In Packer L, Fuchs J (eds) Biothiols in health and disease. Marcel Dekker Inc, New York, p 117–34
Saravanan G, Ponmurugan P (2012) Antidiabetic effect of S-allylcysteine: effect on thyroid hormone and circulatory antioxidant system in experimental diabetic rats. J Diab Comp 26:280–285
Orhan DD, Aslan M, Sendogdu N, Ergun F (2005) Evaluation of the hypoglycemic effect and antioxidant activity of three Viscum album subspecies (European mistletoe) in streptozotocin-diabetic rats. J Ethnopharmacol 98:95–102
Muruganandan S, Gupta S, Kataria M (2002) Mangiferin protects the streptozotocin-induced oxidative damage to cardiac and renal tissues in rats. Toxicology 176:165–73
Ewis SA, Abdel Rahman MS (1995) Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. J App Toxicol 15:387–390
Acknowledgements
The authors thank the Department of Science and Technology, India, for providing financial assistance for this work (Ref No: SR/SO/HS/227/2012). The authors thank the management of K.S.Rangasamy College of Arts and Science, Tiruchengode, India, for providing all the facilities to execute this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Parim Brahmanaidu and V. V. Sathibabu Uddandrao have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Brahmanaidu, P., Uddandrao, V.V.S., Sasikumar, V. et al. Reversal of endothelial dysfunction in aorta of streptozotocin-nicotinamide-induced type-2 diabetic rats by S-Allylcysteine. Mol Cell Biochem 432, 25–32 (2017). https://doi.org/10.1007/s11010-017-2994-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-2994-0