Abstract
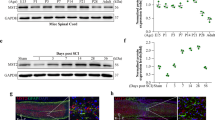
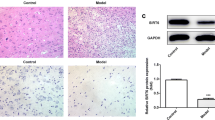
Neuronal cell death following spinal cord injury (SCI) is an important contributor to neurological deficits. The purpose of our work was to delineate the function of mammalian sterile 20-like kinase 1 (Mst1), a pro-apoptotic kinase and key mediator of apoptotic signaling, in the pathogenesis of an experimental mouse model of SCI. Male mice received a mid-thoracic spinal contusion injury, and it was found that phosphorylation of Mst1 at the injured site was enhanced significantly following SCI. Furthermore, when compared to the wild-type controls, Mst1-deficient mice displayed improved locomotor function by increased Basso mouse scale score. Deletion of Mst1 in mice attenuated loss of motor neurons and suppressed microglial and glial activation following SCI. Deletion of Mst1 in mice reduced apoptosis via suppressing cytochrome c release and caspase-3 activation following SCI. Deletion of Mst1 attenuated mitochondrial dysfunction and increased ATP formation following SCI. Deletion of Mst1 in mice inhibited local inflammation following SCI, evidenced by reduced activities of myeloperoxidase and protein levels of TNF-α, IL-1β, and IL-6. In conclusion, the present study demonstrated that deletion of Mst1 attenuated neuronal loss and improved locomotor function in a mouse model of SCI, via preserving mitochondrial function, attenuating mitochondria-mediated apoptotic pathway, and suppressing inflammation, at least in part.








Similar content being viewed by others
Abbreviations
- AFU:
-
Arbitrary fluorescence unit
- BMS:
-
Basso mouse scale
- GFAP:
-
Glial fibrillary acidic protein
- IBA1:
-
Ionized calcium-binding adapter molecule 1
- MMP:
-
Mitochondrial membrane potential
- MPO:
-
Myeloperoxidase
- Mst1:
-
Mammalian sterile 20-like kinase 1
- SCI:
-
Spinal cord injury
- TNF-α:
-
Tumor necrosis factor alpha
- WT:
-
Wild-type mice
References
Chiu WT, Lin HC, Lam C, Chu SF, Chiang YH, Tsai SH (2010) Review paper: epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac J Public Health 22:9–18
Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS (1997) Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 3:73–76
Visavadiya NP, Patel SP, VanRooyen JL, Sullivan PG, Rabchevsky AG (2015) Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol 8:59–67
Faden AI, Wu J, Stoica BA, Loane DJ (2016) Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol 173:681–691
Ling P, Lu TJ, Yuan CJ, Lai MD (2008) Biosignaling of mammalian Ste20-related kinases. Cell Signal 20:1237–1247
Avruch J, Zhou D, Fitamant J, Bardeesy N, Mou F, Barrufet LR (2012) Protein kinases of the Hippo pathway: regulation and substrates. Semin Cell Dev Biol 23:770–784
Yuan F, Xie Q, Wu J, Bai Y, Mao B, Dong Y, Bi W, Ji G, Tao W, Wang Y, Yuan Z (2011) MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. J Biol Chem 286:6940–6945
Graves JD, Gotoh Y, Draves KE, Ambrose D, Han DK, Wright M, Chernoff J, Clark EA, Krebs EG (1998) Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J 17:2224–2234
Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A (2006) A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125:987–1001
Yuan Z, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A (2009) Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem 284:11285–11292
Lee JK, Shin JH, Hwang SG, Gwag BJ, McKee AC, Lee J, Kowall NW, Ryu H, Lim DS, Choi EJ (2013) MST1 functions as a key modulator of neurodegeneration in a mouse model of ALS. Proc Natl Acad Sci USA 110:12066–12071
Zhao J, Chen C, Xiao JR, Wei HF, Zhou XH, Mao XX, Zhang WD, Qian R, Chen XL, He MQ, Yu XW, Zhao J (2015) An up-regulation of IRF-1 after a spinal cord injury: implications for neuronal apoptosis. J Mol Neurosci 57:595–604
Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, Stokes BT (2000) Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma 17:299–319
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG (2006) Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635–659
Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, Takano T, Tian GF, Goldman SA, Nedergaard M (2009) Systemic administration of an antagonist of the ATP-sensitive receptor P2 × 7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA 106:12489–12493
Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG (2009) Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res 87:130–140
Sullivan PG, Dube C, Dorenbos K, Steward O, Baram TZ (2003) Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol 53:711–717
Mullane KM, Kraemer R, Smith B (1985) Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 14:157–167
Liu R, Zhao W, Zhao Q, Liu SJ, Liu J, He M, Xu Y, Wang W, Liu W, Xia QJ, Li CY, Wang TH (2014) Endoplasmic reticulum protein 29 protects cortical neurons from apoptosis and promoting corticospinal tract regeneration to improve neural behavior via caspase and Erk signal in rats with spinal cord transection. Mol Neurobiol 50:1035–1048
Sun F, Kanthasamy A, Song C, Yang Y, Anantharam V, Kanthasamy AG (2008) Proteasome inhibitor-induced apoptosis is mediated by positive feedback amplification of PKCδ proteolytic activation and mitochondrial translocation. J Cell Mol Med 12:2467–2481
McEwen ML, Springer JE (2005) A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J Histochem Cytochem 53:809–819
Yu WR, Liu T, Fehlings TK, Fehlings MG (2009) Involvement of mitochondrial signaling pathways in the mechanism of Fas-mediated apoptosis after spinal cord injury. Eur J Neurosci 29:114–131
Wu KL, Hsu C, Chan JY (2009) Nitric oxide and superoxide anion differentially activate poly(ADP-ribose) polymerase-1 and Bax to induce nuclear translocation of apoptosis-inducing factor and mitochondrial release of cytochrome c after spinal cord injury. J Neurotrauma 26:965–977
Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, Allis CD (2003) Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113:507–517
Vichalkovski A, Gresko E, Cornils H, Hergovich A, Schmitz D, Hemmings BA (2008) NDR kinase is activated by RASSF1A/MST1 in response to Fas receptor stimulation and promotes apoptosis. Curr Biol 18:889–1895
Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, Li H, Hsu CP, Sadoshima J (2014) Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol Cell 54:639–650
Ardestani A, Paroni F, Azizi Z, Kaur S, Khobragade V, Yuan T, Frogne T, Tao W, Oberholzer J, Pattou F, Kerr Conte J, Maedler K (2014) MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat Med 20:385–397
Jia ZQ, Li G, Zhang ZY, Li HT, Wang JQ, Fan ZK, Lv G (2016) Time representation of mitochondrial morphology and function after acute spinal cord injury. Neural Regen Res 11:137–143
Li G, Jia Z, Cao Y, Wang Y, Li H, Zhang Z, Bi J, Lv G, Fan Z (2015) Mitochondrial division inhibitor 1 ameliorates mitochondrial injury, apoptosis, and motor dysfunction after acute spinal cord injury in rats. Neurochem Res 40:1379–1392
Pandya JD, Grondin R, Yonutas HM, Haghnazar H, Gash DM, Zhang Z, Sullivan PG (2015) Decreased mitochondrial bioenergetics and calcium buffering capacity in the basal ganglia correlates with motor deficits in a nonhuman primate model of aging. Neurobiol Aging 36:1903–1913
Semple BD (2014) Early preservation of mitochondrial bioenergetics supports both structural and functional recovery after neurotrauma. Exp Neurol 261:291–297
Wu KL, Hsu C, Chan JY (2007) Impairment of the mitochondrial respiratory enzyme activity triggers sequential activation of apoptosis-inducing factor-dependent and caspase-dependent signaling pathways to induce apoptosis after spinal cord injury. J Neurochem 101:1552–1566
Hu J, Man W, Shen M, Zhang M, Lin J, Wang T, Duan Y, Li C, Zhang R, Gao E, Wang H, Sun D (2016) Luteolin alleviates post-infarction cardiac dysfunction by up-regulating autophagy through Mst1 inhibition. J Cell Mol Med 20:147–156
Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209:378–388
Zhao S, Yin J, Zhou L, Yan F, He Q, Huang L, Peng S, Jia J, Cheng J, Chen H, Tao W, Ji X, Xu Y, Yuan Z (2016) Hippo/MST1 signaling mediates microglial activation following acute cerebral ischemia-reperfusion injury. Brain Behav Immun 55:236–248
Salojin KV, Hamman BD, Chang WC, Jhaver KG, Al-Shami A, Crisostomo J, Wilkins C, Digeorge-Foushee AM, Allen J, Patel N, Gopinathan S, Zhou J, Nouraldeen A, Jessop TC, Bagdanoff JT, Augeri DJ, Read R, Vogel P, Swaffield J, Wilson A, Platt KA, Carson KG, Main A, Zambrowicz BP, Oravecz T (2014) Genetic deletion of Mst1 alters T cell function and protects against autoimmunity. PLoS One 9:e98151
Author contributions
Conception and design of the experiments: P. W., D. X., S. X. Performed the experiments: P. W., D. X., Y. Z., X. L., Y. X., P. Z., Q. F., S. X. Collection, analysis and interpretation of data: P. W., D. X., Y. Z., X. L., Y. X., P. Z., Q. F. Drafting the article: P. W., D. X., S. X.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest disclosure
The authors declare that there is no conflict of interest.
Additional information
Pan-feng Wang and Da-yuan Xu have equally contributed on this work.
Rights and permissions
About this article
Cite this article
Wang, Pf., Xu, Dy., Zhang, Y. et al. Deletion of mammalian sterile 20-like kinase 1 attenuates neuronal loss and improves locomotor function in a mouse model of spinal cord trauma. Mol Cell Biochem 431, 11–20 (2017). https://doi.org/10.1007/s11010-017-2969-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-2969-1