Abstract
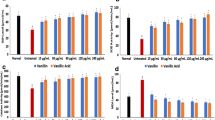
Diorganoyl dichalcogenide compouds can have antioxidant activity in different in vitro and in vivo models. Here, we have compared the potential antioxidant activity of 1-dinaphthyl diselenide (1-NapSe)2, 2-dinaphthyl diselenide (2-NapSe)2, 1-dinaphthyl distelluride (1-NapTe)2, 2-dinaphthyl ditelluride (2-NapTe)2 with their well-studied analogs diphenyl diselenide ((PhSe)2) and diphenyl telluride ((PhTe)2). (PhSe)2, (PhTe)2, and naphthalene analogs-inhibited Fe(II)-induced lipid peroxidation, catalytically decomposed hydrogen peroxide and oxidized thiols, such as dithiothreitol (DTT), Cysteine (CYS), dimercaptopropionic acid (DMPS), and thiophenol (PhSH). (PhSe)2 was the less potent of the tested compounds against Fe(II)-induced lipid peroxidation in brain homogenates and the change in the organic moiety from an aryl to naphthyl group increased considerably the antioxidant potency of diselenide compounds. However, the change from aryl to naphthyl had little effect on the thio-peroxidase-like activity of diorganoyl dichalcogenides. These results suggest that minor changes in the organic moiety of aromatic diselenide compounds can modify profoundly their capacity to inhibit iron-induced lipid peroxidation. The pharmacological properties of organochalcogens are thought to be linked to their capacity of modulating oxidative stress. Consequently, it becomes important to explore the toxicological properties of dinaphthyl diselenides and ditellurides.








Similar content being viewed by others
References
Muller A, Cadenas E, Graf P, Sies HA (1984) Novel biologically active seleno-organic compound I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ51 (Ebselen). Biochem Pharmacol 33:3235–3239
Nogueira CW, Rocha JBT (2011) Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds. Arch Toxicol 85:1313–1359
Kumar S, Engman L, Valgimigli L, Amorati R, Fumo M, Pedulli GF (2007) Antioxidant profile of ethoxyquin and some of its S, Se, and Te analogues. J Org Chem 72:6046–6055
de Souza Prestes A, Stefanello ST, Salman SM, Pazini AM, Schwab RS, Braga AL, de Vargas Barbosa NB, Rocha JBT (2012) Antioxidant activity of β-selenoamines and their capacity to mimic different enzymes. Mol Cell Biochem 365:85–92
de Freitas AS, Rocha JB (2011) Diphenyl diselenide and analogs are substrates of cerebral rat thioredoxin reductase: a pathway for their neuroprotective effects. Neurosci Lett 503:1–5
Zhao R, Holmgren A (2002) A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem 278:39456–39462
Sausen de Freitas A, de Souza Prestes A, Wagner C, Sudati JH, Alves D, Oliveira Porciúncula L, Kade IJ, Rocha JBT (2010) Reduction of diphenyl diselenide and analogs by mammalian thioredoxin reductase is independent of their gluthathione peroxidase-like activity: a possible novel pathway for their antioxidant activity. Molecules 15:7699–7714
Engman L, Persson J, Vessman K, Ekstrom M, Berglund M, Andersson C (1995) Organotellurium compounds as efficient retards of lipid peroxidation in methanol. Free Rad Biol Med 19:441–452
Avila DS, Gubert P, Palma A, Colle D, Alves D, Nogueira CW, Rocha JBT, Soares FA (2008) An organotellurium compound with antioxidant activity against excitotoxic agents without neurotoxic effects in brain of rats. Brain Res Bull 76:114–123
Avila DS, Colle D, Gubert P, Palma AS, Puntel G, Manarin F, Noremberg S, Nascimento PC, Aschner M, Rocha JB, Soares FA (2010) A possible neuroprotective action of a vinylic telluride against Mn-induced neurotoxicity. Toxicol Sci 115:194–201
Moretto MB, Boff B, Franco J, Posser T, Roessler TM, Souza DO, Nogueira CW, Wofchuk S, Rocha JBT (2007) Ca-45(2 +) influx in rat brain: effect of diorganylchalcogenides compounds. Toxicol Sci 99:566–571
Ardais AP, Viola GG, Costa MS, Nunes F, Behr GA, Klamt F, Moreira JC, Souza DO, Rocha JB, Porciúncula LO (2010) Acute treatment with diphenyl diselenide inhibits glutamate uptake into rat hippocampal slices and modifies glutamate transporters, SNAP-25, and GFAP immunocontent. Toxicol Sci 113:434–443
Lopes FM, Londero GF, de Medeiros LM, da Motta LL, Behr GA, de Oliveira VA, Ibrahim M, Moreira JC, de Oliveira Porciúncula L, da Rocha JB, Klamt F (2012) Evaluation of the neurotoxic/neuroprotective role of organoselenides using differentiated human neuroblastoma SH-SY5Y cell line challenged with 6-hydroxydopamine. Neurotox Res 22:138–149
Ibrahim M, Prigol M, Hassan W, Nogueira CW, Zeni G, Rocha JBT (2010) Protective effect of binaphthyl diselenide, a synthetic organoselenium compound, on 2-nitropropane-induced hepatotoxicity in rats. Cell Biochem Funct 28:1–9
Puntel RL, Roos DH, Folmer V, Nogueira CW, Galina A, Aschner M, Rocha JBT (2010) Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening. Toxicol Sci 117:133–143
Jomova K, Vondrakova D, Lawson M, Valko M (2010) Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345:91–110
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. ChemBiol Interact 160:1–40
Franco R, Sanchez-Olea RR, Reyes-Reyes EM, Panayiotidis MI (2009) Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mut Res (Gen Tox Env Mut) 674:3–22
Migliore L, Coppede F (2009) Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mut Res Gen Tox Env Mut 674:73–84
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Gonçalves TL, Benvegnú DM, Bonfanti G, Frediani AV, Rocha JB (2009) Delta-ALA-D activity is a reliable marker for oxidative stress in bone marrow transplant patients. BMC Cancer 9:138
Kade IJ, Paixao MW, Rodrigues OED, Barbosa NBV, Braga AL, Avila DS, Nogueira CW, Rocha JBT (2008) Comparative studies on dicholesteroyl diselenide and diphenyl diselenide as antioxidant agents and their effect on the activities of Na +/K + ATPase and delta-aminolevulinic acid dehydratase in the rat brain. Neurochem Res 33:167–178
Kade IJ, Rocha JBT (2010) Comparative study on the influence of subcutaneous administration of diphenyl and dicholesteroyl diselenides on sulfhydryl proteins and antioxidant parameters in mice. J Appl Toxicol 30:688–693
Nogueira CW, Meotti FC, Curte E, Pilissao C, Zeni G, Rocha JBT (2003) Investigations into the potential neurotoxicity induced by diselenides in mice and rats. Toxicology 183:29–37
Paulmier C (1986) Synthesis and properties of selenides. In: Baldwin JE (ed) Selenium reagents and intermediates in organic synthesis. Pergamon Press, Oxford, pp 84–116
Ohkawa H, Ohishi YNK (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Puntel RL, Roos DH, Grotto D, Garcia SC, Nogucira CW, Rocha JBT (2007) Antioxidant properties of Krebs cycle intermediates against malonate pro-oxidant activity in vitro: a comparative study using the colorimetric method and HPLC analysis to determine malondialdehyde in rat. Life Sci 81:51–62
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root; their relative astringency and radical scavenging effects. Chem Pharm Bul 36:2090–2097
Iwaoka M, Tomoda S (1994) A model study on the effect of an amino group on the antioxidant activity of glutathione peroxidase. J Am Chem Soc 116:2557–2561
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Anderson CM, Hallberg A, Brattsand R, Cotgreave IA, Engman L, Person J (1993) Glutathione peroxidase-like activity of diaryl tellurides. Bioorg Med Chem Lett 3:2553–2558
Anderson CM, Brattsand R, Hallberg AR, Engman L, Persson J, Moldeus P, Cotgreave I (1994) Diaryl tellurides as inhibitors of lipid peroxidation in biological and chemical systems. Free Rad Res 20:401–410
Avila DS, Beque MC, Folmer V, Braga AL, Zeni G, Nogueira CW, Soares FAA, Rocha JBT (2006) Diethyl 2-phenyl-2 tellurophenyl vinylphosphonate: an organotellurium compound with low toxicity. Toxicology 224:100–107
Ibrahim M, Luchese C, Pinton S, Roman SS, Hassan W, Nogueira CW, Rocha JBT (2011) Involvement of catalase in the protective effect of binaphthyl diselenide against renal damage induced by glycerol. Exp Toxicol Pathol 63:331–335
Comparsi B, Meinerz DF, Franco JL, Posser T, Prestes AS, Stefanello ST, dos Santos DB, Wagner C, Farina M, Aschner M, Dafre AL, Rocha JBT (2012) Diphenyl ditelluride targets brain selenoproteins in vivo: inhibition of cerebral thioredoxin reductase and glutathione peroxidase in mice after acute exposure. doi:10.1007/s11010-012-1408-6
Rocha JBT, Saraiva RA, Garcia SC, Gravina F, Nogueira CW (2012) Aminolevulinate dehydratase (δ-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Tox Res 1:85–102
Ibrahim M, Hassan W, Meinerz DF, de Oliveira Leite G, Nogueira CW, Rocha JBT (2012) Ethanol-induced oxidative stress: the role of binaphthyl diselenide as a potentantioxidant. Biol Trace Elem Res 147:309–314
Hassan W, Narayanaperumal S, Gul K, Rahman AU, Braga AL, Rodrigues OD, Rocha JBT (2012) Modulation of diorganoyl dichalcogenides reactivity by non-bonded nitrogen interactions. Chem Biol Interact 199:96–105. doi:10.1016/j.cbi.2012.05.010
Acknowledgments
Mohammad Ibrahim is specifically grateful for the financial support of TWAS and CNPq. M. Ibrahim is a beneficiary of the TWAS-CNPq postgraduate (doctoral) and Matheus Santos (PRAE-UFSM) fellowship program. João B.T. da Rocha gratefully acknowledges the financial support of CNPq, INCT for Excitoxicity and Neuroprotection, IBNet-FINEP, FAPERGS, CAPES, CAPES/SAUX, FAPERS-PRONEX-CNPQ, and VITAE.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibrahim, M., Hassan, W., Meinerz, D.F. et al. Antioxidant properties of diorganoyl diselenides and ditellurides: modulation by organic aryl or naphthyl moiety. Mol Cell Biochem 371, 97–104 (2012). https://doi.org/10.1007/s11010-012-1426-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1426-4