Abstract
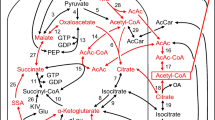
Methyl succinate (MS) and α-ketoisocaproate (KIC) when applied alone to cultured pancreatic islets or INS-1 832/13 cells do not stimulate insulin release. However, when the two metabolites are combined together they strongly stimulate insulin release. Studying the possible explanations for this complementarity has provided clues to the pathways involved in insulin secretion. MS increased carbon incorporation of KIC into acid-precipitable material and lipid in INS-1 cells. In isolated mitochondria, MS alone increased malate, but MS plus KIC increased citrate, α-ketoglutarate, and isocitrate. These data and the known pathways of their metabolism suggest that MS supplies the oxaloacetate component of citrate and KIC supplies the acetate component of citrate. Other citric acid cycle intermediates can be formed from citrate enabling anaplerosis to supply precursors for extramitochondrial pathways. In addition, KIC, glucose and pyruvate can be metabolized to acetoacetate. In an INS-1 cell line deficient in ATP citrate lyase, incorporation of carbon from pyruvate into acid-precipitable material and lipid was not lowered. This negative result is in agreement with our recent discovery that citrate is not the only carrier of acyl groups from the mitochondria to the cytosol in the beta cell and that acetoacetate can also transfer acyl carbon to the cytosol.


Similar content being viewed by others
Abbreviations
- KIC:
-
α-Ketoisocaproate
References
Brunengraber H, Roe CR (2006) Anaplerotic molecules: current and future. J Inherit Metab Dis 29:327–331
MacDonald MJ (1993) Metabolism of the insulin secretagogue methyl succinate by pancreatic islets. Arch Biochem Biophys 300:201–205
MacDonald MJ (1993) Estimates of glycolysis, pyruvate (de)carboxylation, pentose phosphate pathway and methyl succinate metabolism in incapacitated pancreatic islets. Arch Biochem Biophys 305:205–214
MacDonald MJ (1993) Glucose enters mitochondrial metabolism via both carboxylation and decarboxylation of pyruvate in pancreatic islets. Metabolism 42:1229–1231
MacDonald MJ (1995) Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets: further implication of cytosolic NADPH in insulin secretion. J Biol Chem 270:20051–20058
Khan A, Ling ZC, Landau BR (1996) Quantifying the carboxylation of pyruvate in pancreatic islets. J Biol Chem 271:2539–2542
MacDonald MJ (2007) Synergistic potent insulin release by combinations of weak secretagogues in pancreatic islets and INS-1 cells. J Biol Chem 282:6043–6052
MacDonald MJ, Longacre MJ, Stoker SW, Brown LJ, Hasan NM, Kendrick MA (2008) Acetoacetate and β-hydroxybutyrate in combination with other metabolites release insulin from INS-1 cells and provide clues about pathways in insulin secretion. Am J Phys Cell Phys 294:C442–C450
Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB (2000) Isolation of INS-1-derived cell lines with robust ATP- sensitive K+ channel-dependent and –independent glucose-stimulated insulin secretion. Diabetes 494:24–30
Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167–178
MacDonald MJ (2004) Production and export of metabolites from liver and heart mitochondria and anaplerosis. Mol Cell Biochem 258:201–210
MacDonald MJ (2003) The export of metabolites from mitochondria and anaplerosis in insulin secretion. Biochim Biophys Acta 1619:77–88
Farfari S, Schulz V, Corkey B, Prentki M (2000) Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49:718–726
Flamez D, Berger V, Kruhoffer M, Orntoft T, Pipeleers D, Schuit FC (2002) Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes 51:2018–2024
MacDonald MJ, Smith AD, Hasan NM, Sabat G, Fahein LA (2007) Feasibility of pathways for transport of acyl groups from mitochondria to the cytosol to form short chain acyl-CoAs in the pancreatic beta cell. J Biol Chem 282:30596–30606
MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA (2005) Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab 288:E1–E15
Acknowledgements
This work was supported by NIH Grant DK28348 and by a gift from the Oscar C. Rennebohm Foundation. The author thanks Julian Buss and Melissa Longacre for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MacDonald, M.J., Stoker, S.W. & Hasan, N.M. Anaplerosis from glucose, α-ketoisocaproate, and pyruvate in pancreatic islets, INS-1 cells and liver mitochondria. Mol Cell Biochem 313, 195–202 (2008). https://doi.org/10.1007/s11010-008-9757-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9757-x