Abstract
Objectives
(1) To evaluate the direct (un-mediated) and indirect (mediated) relationship between antenatal exposure to opioid agonist medication as treatment for opioid use disorder (MOUD) and the severity of neonatal opioid withdrawal syndrome (NOWS), and (2) to understand the degree to which mediating factors influence the direct relationship between MOUD exposure and NOWS severity.
Methods
This cross-sectional study includes data abstracted from the medical records of 1294 opioid-exposed infants (859 MOUD exposed and 435 non-MOUD exposed) born at or admitted to one of 30 US hospitals from July 1, 2016, to June 30, 2017. Regression models and mediation analyses were used to evaluate the relationship between MOUD exposure and NOWS severity (i.e., infant pharmacologic treatment and length of newborn hospital stay (LOS)) to identify potential mediators of this relationship in analyses adjusted for confounding factors.
Results
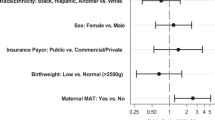
A direct (un-mediated) association was found between antenatal exposure to MOUD and both pharmacologic treatment for NOWS (aOR 2.34; 95%CI 1.74, 3.14) and an increase in LOS (1.73 days; 95%CI 0.49, 2.98). Delivery of adequate prenatal care and a reduction in polysubstance exposure were mediators of the relationship between MOUD and NOWS severity and as thus, were indirectly associated with a decrease in both pharmacologic treatment for NOWS and LOS.
Conclusions for Practice
MOUD exposure is directly associated with NOWS severity. Prenatal care and polysubstance exposure are potential mediators in this relationship. These mediating factors may be targeted to reduce the severity of NOWS while maintaining the important benefits of MOUD during pregnancy.
Significance
What is already known on this subject? The use of MOUD during pregnancy improves fetal outcomes, while antenatal exposure to MOUD increases the risk of NOWS. The severity of NOWS is influenced by MOUD type, co-exposures, adequacy of prenatal care, and the infant’s gestational age.
What this study adds? This study identifies factors that mediate the direct influence of antenatal MOUD exposure on the severity of NOWS and quantifies the degree to which this mediation influences outcomes in a large and geographically diverse population. Thus, providing clinicians with potential targets to improve care for this vulnerable population.


Similar content being viewed by others
Data Availability
The dataset supporting the conclusions of this article are available by request to the Corresponding Author through a data use agreement. A deidentified dataset including the majority of these data is available via the NICHD DASH portal.
Code Availability
Not applicable.
Abbreviations
- ACT NOW:
-
Advancing Clinical Trials in Neonatal Opioid Withdrawal Syndrome
- APC:
-
Adequate prenatal care
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- CE:
-
Current experience
- ECHO:
-
Environmental influences on Child Health Outcomes
- GA:
-
Gestational age
- HEAL:
-
Helping to End Addiction Long-term
- IRB:
-
Institutional Review Board
- ISPCTN:
-
IDeA States Pediatric Clinical Trials Network
- LOS:
-
Length of stay
- MOUD:
-
Medication for opioid use disorder
- NICHD:
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NIH:
-
National Institutes of Health
- NOWS:
-
Neonatal opioid withdrawal syndrome
- NRN:
-
Neonatal Research Network
- OR:
-
Odds ratio
- OUD:
-
Opioid use disorder
- RUCA:
-
Rural–urban commuting area
- SD:
-
Standard deviation
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
References
American College of Obstetrics and Gynecologists. (2017). ACOG Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstetrics & Gynecology, 130(2), e81–e94. https://doi.org/10.1097/AOG.0000000000002235
Coyle, M. G., Brogly, S. B., Ahmed, M. S., Patrick, S. W., & Jones, H. E. (2018). Neonatal abstinence syndrome. Nature Reviews Disease Primers, 4(1), 47. https://doi.org/10.1038/s41572-018-0045-0
Ecker, J., Abuhamad, A., Hill, W., Bailit, J., Bateman, B. T., Berghella, V., Blake-Lamb, T., Guille, C., Landau, R., Minkoff, H., Prabhu, M., Rosenthal, E., Terplan, M., Wright, T. E., & Yonkers, K. A. (2019). Substance use disorders in pregnancy: Clinical, ethical, and research imperatives of the opioid epidemic: A report of a joint workshop of the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, and American Society of Addiction Medicine. American Journal of Obstetrics and Gynecology, 221(1), B5–B28. https://doi.org/10.1016/j.ajog.2019.03.022
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Neonatal Research Network. (2017). Survey of Morbidity and Mortality Among High Risk Preterm Infants (GDB). Retrieved from https://neonatal.rti.org/pdf/GDBPublic/Public_GDB_Manual.pdf
Grossman, M. R., Lipshaw, M. J., Osborn, R. R., & Berkwitt, A. K. (2018). A novel approach to assessing infants with Neonatal Abstinence Syndrome. Hospital Pediatrics, 8(1), 1–6. https://doi.org/10.1542/hpeds.2017-0128
Haight, S. C., Ko, J. Y., Tong, V. T., Bohm, M. K., & Callaghan, W. M. (2018). Opioid use disorder documented at delivery hospitalization—United States, 1999–2014. Morbidity and Mortality Weekly Report, 67(31), 845–849. https://doi.org/10.15585/mmwr.mm6731a1
Huybrechts, K. F., Bateman, B. T., Desai, R. J., Hernandez-Diaz, S., Rough, K., Mogun, H., Kerzner, L. S., Davis, J. M., Stover, M., Bartels, D., Cottral, J., & Patorno, E. (2017). Risk of neonatal drug withdrawal after intrauterine co-exposure to opioids and psychotropic medications: Cohort study. BMJ, 358, j3326. https://doi.org/10.1136/bmj.j3326
Hwang, S. S., Weikel, B., Adams, J., Bourque, S. L., Cabrera, J., Griffith, N., Hall, A. M., Scott, J., Smith, D., Wheeler, C., Woodard, J., & Wymore, E. (2020). The Colorado Hospitals substance exposed newborn quality improvement collaborative: Standardization of care for opioid-exposed newborns shortens length of stay and reduces number of infants requiring opiate therapy. Hospital Pediatrics, 10(9), 783–791. https://doi.org/10.1542/hpeds.2020-0032
Jansson, L. M., Di Pietro, J. A., Elko, A., Williams, E. L., Milio, L., & Velez, M. (2012). Pregnancies exposed to methadone, methadone and other illicit substances, and poly-drugs without methadone: A comparison of fetal neurobehaviors and infant outcomes. Drug and Alcohol Dependence, 122(3), 213–219. https://doi.org/10.1016/j.drugalcdep.2011.10.003
Jones, H. E., Kaltenbach, K., Heil, S. H., Stine, S. M., Coyle, M. G., Arria, A. M., O’Grady, K. E., Selby, P., Martin, P. R., & Fischer, G. (2010). Neonatal abstinence syndrome after methadone or buprenorphine exposure. The New England Journal of Medicine, 363(24), 2320–2331. https://doi.org/10.1056/NEJMoa1005359
Jones, H. E., Heil, S. H., Baewert, A., Arria, A. M., Kaltenbach, K., Martin, P. R., Coyle, M. G., Selby, P., Stine, S. M., & Fischer, G. (2012). Buprenorphine treatment of opioid-dependent pregnant women: A comprehensive review. Addiction, 107(Suppl 1), 5–27. https://doi.org/10.1111/j.1360-0443.2012.04035.x
Krans, E. E., Kim, J. Y., Chen, Q., Rothenberger, S. D., James, A. E., III., Kelley, D., & Jarlenski, M. P. (2021). Outcomes associated with the use of medications for opioid use disorder during pregnancy. Addiction, 116(12), 3504–3514. https://doi.org/10.1111/add.15582
Kreek, M. J., Bart, G., Lilly, C., LaForge, K. S., & Nielsen, D. A. (2005). Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacological Reviews, 57(1), 1–26. https://doi.org/10.1124/pr.57.1.1
Lacroix, I., Berrebi, A., Garipuy, D., Schmitt, L., Hammou, Y., Chaumerliac, C., Lapeyre-Mestre, M., Montastruc, J. L., & Damase-Michel, C. (2011). Buprenorphine versus methadone in pregnant opioid-dependent women: A prospective multicenter study. European Journal of Clinical Pharmacology, 67(10), 1053–1059. https://doi.org/10.1007/s00228-011-1049-9
Lee, H., Herbert, R. D., & McAuley, J. H. (2019). Mediation analysis. JAMA, 321(7), 697–698. https://doi.org/10.1001/jama.2018.21973
Ludlow, J. P., Evans, S. F., & Hulse, G. (2004). Obstetric and perinatal outcomes in pregnancies associated with illicit substance abuse. The Australian and New Zealand Journal of Obstetrics and Gynaecology, 44(4), 302–306. https://doi.org/10.1111/j.1479-828X.2004.00221.x
Lynch, C. D., & Prasad, M. R. (2018). Causal analysis in evaluating complex health interventions: Identifying the optimal treatment for opioid abuse in pregnancy. Paediatric and Perinatal Epidemiology, 32(2), 223–224. https://doi.org/10.1111/ppe.12464
Mackinnon, D. P., & Dwyer, J. H. (1993). Estimating mediated effects in prevention studies. Evaluation Review, 17(2), 144–158. https://doi.org/10.1177/0193841X9301700202
Mascha, E. J., Dalton, J. E., Kurz, A., & Saager, L. (2013). Understanding the mechanism: Mediation analysis in randomized and nonrandomized studies. Anesthesia & Analgesia, 117(4), 980–994. https://doi.org/10.1213/ANE.0b013e3182a44cb9
Minozzi, S., Amato, L., Bellisario, C., Ferri, M., & Davoli, M. (2013). Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD006318.pub3
National Center for Complementary and Integrative Health. (2015). Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. Retrieved from https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html. Accessed Jan 3, 2022
Parikh, A., Gopalakrishnan, M., Azeem, A., Booth, A., & El-Metwally, D. (2019). Racial association and pharmacotherapy in neonatal opioid withdrawal syndrome. Journal of Perinatology, 39(10), 1370–1376. https://doi.org/10.1038/s41372-019-0440-8
Patrick, S. W., Barfield, W. D., Poindexter, B. B., Committee on Fetus and Newborn, Committee on Substance Use and Prevention, Cummings, J., Hand, I., Adams-Chapman, I., Aucott, S. W., Puopolo, K. M., Goldsmith, J. P., Kaufman, D., Martin, C., Mowitz, M., Gonzalez, L., Camenga, D. R., Quigley, J., Ryan, S. A., & Walker-Harding, L. (2020). Neonatal Opioid Withdrawal Syndrome. Pediatrics. https://doi.org/10.1542/peds.2020-029074
Reddy, U. M., Davis, J. M., Ren, Z., & Greene, M. F. (2017). Opioid Use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: Executive Summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstetrics & Gynecology, 130(1), 10–28. https://doi.org/10.1097/AOG.0000000000002054. Accessed Jan 3, 2022
Sanlorenzo, L. A., Cooper, W. O., Dudley, J. A., Stratton, S., Maalouf, F. I., & Patrick, S. W. (2019). Increased severity of neonatal abstinence syndrome associated with concomitant antenatal opioid and benzodiazepine exposure. Hospital Pediatrics, 9(8), 569–575. https://doi.org/10.1542/hpeds.2018-0227
Smirk, C. L., Bowman, E., Doyle, L. W., & Kamlin, C. O. (2014). How long should infants at risk of drug withdrawal be monitored after birth? Journal of Paediatrics and Child Health, 50(5), 352–355. https://doi.org/10.1111/jpc.12513
Tolia, V. N., Murthy, K., Bennett, M. M., Miller, E. S., Benjamin, D. K., Smith, P. B., & Clark, R. H. (2018). Antenatal methadone vs buprenorphine exposure and length of hospital stay in infants admitted to the intensive care unit with neonatal abstinence syndrome. Journal of Perinatology, 38(1), 75–79. https://doi.org/10.1038/jp.2017.157
United States Department of Agriculture Economic Research Service. (2019). Rural-Urban Commuting Area (RUCA) Codes. Retrieved from https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. Accessed Jan 3, 2022
Villapiano, N. L., Winkelman, T. N., Kozhimannil, K. B., Davis, M. M., & Patrick, S. W. (2017). Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatrics, 171(2), 194–196. https://doi.org/10.1001/jamapediatrics.2016.3750
Wachman, E. M., Grossman, M., Schiff, D. M., Philipp, B. L., Minear, S., Hutton, E., Saia, K., Nikita, F., Khattab, A., Nolin, A., Alvarez, C., Barry, K., Combs, G., Stickney, D., Driscoll, J., Humphreys, R., Burke, J., Farrell, C., Shrestha, H., & Whalen, B. L. (2018). Quality improvement initiative to improve inpatient outcomes for Neonatal Abstinence Syndrome. Journal of Perinatology, 38, 1114–1122. https://doi.org/10.1038/s41372-018-0109-8
Wachman, E. M., Newby, P. K., Vreeland, J., Byun, J., Bonzagni, A., Bauchner, H., & Philipp, B. L. (2011). The relationship between maternal opioid agonists and psychiatric medications on length of hospitalization for neonatal abstinence syndrome. Journal of Addiction Medicine, 5(4), 293–299. https://doi.org/10.1097/ADM.0b013e3182266a3a
Whiteman, V. E., Salemi, J. L., Mogos, M. F., Cain, M. A., Aliyu, M. H., & Salihu, H. M. (2014). Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. Journal of Pregnancy, 2014, 906723. https://doi.org/10.1155/2014/906723
Winer, E. S., Cervone, D., Bryant, J., McKinney, C., Liu, R. T., & Nadorff, M. R. (2016). Distinguishing mediational models and analyses in clinical psychology: Atemporal associations do not imply causation. Journal of Clinical Psychology, 72(9), 947–955. https://doi.org/10.1002/jclp.22298
Young, L. W., Hu, Z., Annett, R. D., Das, A., Fuller, J. F., Higgins, R. D., Lester, B. M., Merhar, S. L., Simon, A. E., Ounpraseuth, S., Smith, P. B., Crawford, M. M., Atz, A. M., Cottrell, L. E., Czynski, A. J., Newman, S., Paul, D. A., Sanchez, P. J., Semmens, E. O., …, Devlin, L. A. (2021). Site-level variation in the characteristics and care of infants with Neonatal Opioid Withdrawal. Pediatrics. https://doi.org/10.1542/peds.2020-008839
Zozus, M. N., Young, L. W., Simon, A. E., Garza, M., Lawrence, L., Ounpraseuth, S. T., Bledsoe, M., Newman-Norlund, S., Jarvis, J. D., McNally, M., Harris, K. R., McCulloh, R., Aikman, R., Cox, S., Malloch, L., Walden, A., Snowden, J., Chedjieu, I. M., Wicker, C. A., …, Devlin, L. A. (2019). Training as an intervention to decrease medical record abstraction errors multicenter studies. Studies in Health Technology and Informatics, 257, 526–539.
Acknowledgements
The NIH, the NICHD, and the National Center for Advancing Translational Sciences (NCATS) provided support for the NRN. The NIH, Office of the Director, ECHO program provided support for the ISPCTN. We are indebted to our medical and nursing colleagues from the ISPCTN and NRN who participated in this study detailed in Online Resource 8.
Funding
This manuscript is the product of work from the NICHD Neonatal Research Network and the ECHO IDeA States Pediatric Clinical Trials Network. Both networks are cooperative agreements with the NIH. Only NIH staff listed as authors have contributed to this manuscript. This research was supported through the NIH HEAL Initiative under award numbers: U10 HD36790, U10 HD53089, U10 HD27904, U24OD024957, U2COD023375, UG1 HD21364, UG1 HD27853, UG1 HD68278, UG1OD024942, UG1OD024943, UG1OD024944, UG1OD024945, UG1OD024946, UG1OD024947, UG1OD024948, UG1OD024949, UG1OD024950, UG1OD024951, UG1OD024952, UG1OD024953, UG1OD024954, UG1OD024955, UG1OD024956, UG1OD024958, UG1OD024959, UL1 TR41. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Consortia
Contributions
LAD: contributed to the design, methodology, investigation, writing and drafting of the initial manuscript and additional editing, ZH: contributed to the methodology, formal analysis, writing and drafting of the initial manuscript and additional editing, SO: contributed to the methodology, formal analysis, writing and drafting of the initial manuscript and additional editing, AES: contributed to the design, methodology, writing and drafting of the initial manuscript and additional editing, RDA: investigation, review and editing of the manuscript, AD: contributed to the design, methodology, writing and drafting of the initial manuscript and additional editing, JFF: investigation, review and editing of the manuscript, RDH: contributed to the design, methodology, writing and drafting of the initial manuscript and additional editing, SLM: contributed to the design, methodology, writing and drafting of the initial manuscript and additional editing, PBS: contributed to the design, methodology, writing and drafting of the initial manuscript and additional editing, MMC: investigation and review and editing of the manuscript, LEC: investigation and review and editing of the manuscript, AJC: investigation and review and editing of the manuscript, SN: investigation and review and editing of the manuscript, DAP: investigation and review and editing of the manuscript, PJS: investigation and review and editing of the manuscript, EOS: investigation and review and editing of the manuscript, MCS: investigation and review and editing of the manuscript, BLW: design, methodology, writing and drafting of the initial manuscript and additional editing, JNS: design, methodology, investigation, writing and drafting of the initial manuscript and additional editing, LWY: contributed to the design, methodology, investigation, writing and drafting of the initial manuscript and additional editing. All authors gave approval for the version of the manuscript that was submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose. While NICHD and NIH ECHO staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD or the ECHO program, the National Institutes of Health, the Department of Health and Human Services, or the US Government.
Ethical Approval
A central Institutional Review Board (IRB) at the University of Arkansas for Medical Sciences approved this chart review study under a waiver of consent and parental permission.
Consent to Participate
This study was IRB approved under wavier of consent. All study data are presented as a conglomerate and no individual participant data is included in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devlin, L.A., Hu, Z., Ounpraseuth, S. et al. The Influence of Mediators on the Relationship Between Antenatal Opioid Agonist Exposure and the Severity of Neonatal Opioid Withdrawal Syndrome. Matern Child Health J 27, 1030–1042 (2023). https://doi.org/10.1007/s10995-022-03521-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-022-03521-3