Abstract
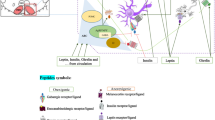
Several mechanisms both inside and outside the central nervous system (CNS) collaborate to control feeding. The hypothalamus received food-related messages from different regions of the brain, and exports appropriate output through specific hypothalamic nuclei. One of the most important of these hypothalamic nuclei is the lateral hypothalamus area (LHA). In this review, multiple valid papers from electronic sources (including Web of Science, Scopus, PubMed, SID, Google Scholar, and ISI databases) were used; which in them the role of LHA in the central regulation of feeding investigated. The hypothalamus is responsible for the central control of food intake in the CNS. The arcuate nucleus, paraventricular nucleus, and LHA are specific regions in the hypothalamus that regulate food intake. The LHA is considered to be the center of hunger or feeding among these nuclei. With the interaction of orexigenic and anorexigenic neurons, as well as various neurotransmitters in several neuronal pathways, this nucleus produces increased food intake. The LHA eventually improved food intake via extensive brain connections. The LHA via various neurons and synaptic connections with other special hypothalamic nuclei involved in the control of nutritional behavior (graphical abstract) stimulated food intake.
Graphical Abstract

Similar content being viewed by others
Abbreviations
- LHA:
-
Lateral hypothalamus area
- PNS:
-
Peripheral nervous system
- ARC:
-
Arcuate nucleus
- PVN:
-
Paraventricular nucleus
- AgRP:
-
Agouti-related protein
- POMC:
-
Pro-opiomelanocortin
- BNTS:
-
Bed nucleus of the stria terminalis
- GABA:
-
Gamma-aminobutyric acid
- GI:
-
Glucose-inhibition
- GE:
-
Glucose-excited
- CART:
-
Cocaine-amphetamine regulated transcript
- BBB:
-
Blood–brain barrier
- GLUTs:
-
Glucose transporters
- SGLTs:
-
Sodium–glucose co-transporters
- NPYR:
-
Neuropeptide Y receptor
- α-MSH:
-
α-Melanocortin stimulating hormone
- MC4R:
-
Melanocortin stimulating 4 receptor
- ICV:
-
Intracerebroventricular injection
- CRH:
-
Corticotropin-releasing hormone
- Lp-R:
-
Leptin receptor
- CNS:
-
Central nervous system
- GPCR:
-
G protein-coupled receptor
- OXR:
-
Orexin receptors
- IR:
-
Insulin receptors
- CAMP:
-
Cyclic adenosine monophosphate
- GHSR1a:
-
Growth hormone secretagogue receptor 1a
- NTs-R:
-
Neurotensin receptor
- GalR1:
-
Galanin-receptor1
References
Berthoud HR, Münzberg H (2011) The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav 104(1):29–39
Björklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30(5):194–202
Brown J, Sagante A, Mayer T, Wright A, Bugescu R, Fuller PM, Leinninger G (2018) Lateral hypothalamic area neurotensin neurons are required for control of orexin neurons and energy balance. Endocrinology 159(9):3158–3176
Brown JA, Wright A, Bugescu R, Christensen L, Olson DP, Leinninger GM (2019) Distinct subsets of lateral hypothalamic neurotensin neurons are activated by leptin or dehydration. Sci Rep 9(1):1–16
Chaillou E, Tillet Y (2005) Nutrition and hypothalamic neuropeptides in sheep: histochemical studies. Histol Histopathol
Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z (2004) Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145(6):2607–2612
Chen Y, Lin YC, Kuo TW, Knight ZA (2015) Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160(5):829–841
Chen Y, Lin YC, Zimmerman CA, Essner RA, Knight ZA (2016) Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife 5:e18640
Clarke RE, Verdejo-Garcia A, Andrews ZB (2018) The role of corticostriatal–hypothalamic neural circuits in feeding behaviour: implications for obesity. J Neurochem 147(6):715–729
Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M (2012) Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. J Comp Neurol 520(18):4168–4183
Farrokhi R, Babapour V, Zendehdel M, Asghari A, Gilanpour H (2020) The role of dopaminergic and cannabinoidergic receptors on ghrelin-induced hypophagia in neonatal chicken. Arch Razi Inst 76(4):941–954
Furuse M, Ando R, Bungo T, Shimojo M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40(5):698–700
Guan HZ, Dong J, Jiang ZY, Chen X (2017) α-MSH influences the excitability of feeding-related neurons in the hypothalamus and dorsal vagal complex of rats. BioMed Res Int 2017:1–9
Gumbs MC, Eggels L, Kool T, Unmehopa UA, van den Heuvel JK, Lamuadni K, Mul JD, la Fleur SE (2020a) Neuropeptide Y signaling in the lateral hypothalamus modulates diet component selection and is dysregulated in a model of diet-induced obesity. Neuroscience 447:28–40
Gumbs MC, Eggels L, Vuuregge AH, Unmehopa UA, Mul JD, la Fleur SE (2020) Effects of Neuropeptide Y administration into the lateral hypothalamus on intake of free-choice high-fat high-sucrose diet components of the male Wistar rat. Nutr Neurosci 25(3):621–630
Hajinezhad MR, Hasanein P, Mokhtarpour A (2018) Nociceptin/orphanin FQ (N/OFQ) receptors are involved in adrenaline-induced feeding behavior in broiler cockerels. Int J Pept Res Ther 24(3):403–407
Hamidi F, Yusefvand S (2017) Role of the hypothalamic arcuate nucleus in regulation of food intake (review study). J Neyshabur Univ Med Sci 5(1):52–65
Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, Nakamoto EM, Kanoski SE (2015) Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. Elife 4:e11190
Hurley SW, Johnson AK (2014) The role of the lateral hypothalamus and orexin in ingestive behavior: a model for the translation of past experience and sensed deficits into motivated behaviors. Front Syst Neurosci 8:216
Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, Bhatti JR (2000) Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes 49(2):177–182
Laque A, Yu S, Qualls-Creekmore E, Gettys S, Schwartzenburg C, Bui K, Rhodes C, Berthoud HR, Morrison CD, Richards BK, Münzberg H (2015) Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol Metab 4(10):706–717
Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF (2011) Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab 14(3):313–323
Liu XH, Morris R, Spiller D, White M, Williams G (2001) Orexin a preferentially excites glucose-sensitive neurons in the lateral hypothalamus of the rat in vitro. Diabetes 50(11):2431–2437
López-Ferreras L, Richard JE, Anderberg RH, Nilsson FH, Olandersson K, Kanoski SE, Skibicka KP (2017) Ghrelin’s control of food reward and body weight in the lateral hypothalamic area is sexually dimorphic. Physiol Behav 176:40–49
Luan X, Sun X, Guo F, Zhang D, Wang C, Ma L, Xu L (2017) Lateral hypothalamic Orexin-A-ergic projections to the arcuate nucleus modulate gastric function in vivo. J Neurochem 143(6):697–707
Marino RA, McDevitt RA, Gantz SC, Shen H, Pignatelli M, Xin W, Wise RA, Bonci A (2020) Control of food approach and eating by a GABAergic projection from lateral hypothalamus to dorsal pons. Proc Natl Acad Sci 117(15):8611–8615
McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW (2000) Effect of intracerebroventricular α-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul 279(2):695–703
Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F (2000) Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16(10):843–857
Mercer RE, Chee MJ, Colmers WF (2011) The role of NPY in hypothalamic mediated food intake. Front Neuroendocrinol 32(4):398–415
Michael NJ, Caron A, Lee CE, Castorena CM, Lee S, Zigman JM, Williams KW, Elmquist JK (2020) Melanocortin regulation of histaminergic neurons via perifornical lateral hypothalamic melanocortin 4 receptors. Mol Metab 35:100956
Myers MG, Olson DP, Low MJ, Elias CF, Ahima RS (2016) Brain regulation of feeding and energy homeostasis. Metabolic syndrome. Springer, Cham, pp 347–368
Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, Izadmehr EM, Tye KM (2016) Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90(6):1286–1298
Ono H (2019) Molecular mechanisms of hypothalamic insulin resistance. Int J Mol Sci 20(6):1317
Opland D, Sutton A, Woodworth H, Brown J, Bugescu R, Garcia A, Christensen L, Rhodes C, Myers M Jr., Leinninger G (2013) Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol Metab 2(4):423–434
Patterson CM, Wong JM, Leinninger GM, Allison MB, Mabrouk OS, Kasper CL, Gonzalez IE, Mackenzie A, Jones JC, Kennedy RT, Myers MG Jr (2015) Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology 156(5):1692–1700
Prinz P, Stengel A (2017) Control of food intake by gastrointestinal peptides: mechanisms of action and possible modulation in the treatment of obesity. J Neurogastroenterol Motil 23(2):180
Qualls-Creekmore E, Münzberg H (2018) Modulation of feeding and associated behaviors by lateral hypothalamic circuits. Endocrinology 159(11):3631–3642
Qualls-Creekmore E, Yu S, Francois M, Hoang J, Huesing C, Bruce-Keller A, Burk D, Berthoud HR, Morrison CD, Münzberg H (2017) Galanin-expressing GABA neurons in the lateral hypothalamus modulate food reward and noncompulsive locomotion. J Neurosci 37(25):6053–6065
Rahmani B, Ghashghayi E, Zendehdel M, Khodadadi M, Hamidi B (2021) The crosstalk between brain mediators regulating food intake behavior in birds: a review. Int J Pept Res Ther 27:2349–2370
Rossi MA, Stuber GD (2018) Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab 27(1):42–56
Routh VH (2010) Glucose sensing neurons in the ventromedial hypothalamus. Sensors 10(10):9002–9025
Sahu A (2003) Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 24(4):225–253
Schick RR, Samsami SO, Zimmermann JP, Eberl TH, Endres CH, Schusdziarra VO, Classen ME (1993) Effect of galanin on food intake in rats: involvement of lateral and ventromedial hypothalamic sites. Am J Physiol Regul Integr Comp Physiol 264(2):355–361
Schroeder LE, Leinninger GM (2018) Role of central neurotensin in regulating feeding: implications for the development and treatment of body weight disorders. Biochim Biophys Acta BBA Mol Basis Dis 1864(3):900–916
Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH (2001) Convergence of pre-and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50(12):2673–2681
Stuber GD, Wise RA (2016) Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 19(2):198–205
Sutcliffe JG, de Lecea L (2002) The hypocretins: setting the arousal threshold. Nat Rev Neurosci 3(5):339–348
Szczypka MS, Rainey MA, Palmiter RD (2000) Dopamine is required for hyperphagia in Lep ob/ob mice. Nat Genet 25(1):102–104
Takeda K, Ono H, Ishikawa K, Ohno T, Kumagai J, Ochiai H, Matumoto A, Yokoh H, Maezawa Y, Yokote K (2021) Central administration of sodium–glucose cotransporter-2 inhibitors increases food intake involving adenosine monophosphate-activated protein kinase phosphorylation in the lateral hypothalamus in healthy rats. BMJ Open Diabetes Res Care 9(1):e002104
Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S (2003) Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 144(4):1506–1512
Van Gestel MA, Kostrzewa E, Adan RA, Janhunen SK (2014) Pharmacological manipulations in animal models of anorexia and binge eating in relation to humans. Br J Pharmacol 171(20):4767–4784
Vucetic Z, Reyes TM (2010) Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med 2(5):577–593
Woodworth HL, Beekly BG, Batchelor HM, Bugescu R, Perez-Bonilla P, Schroeder LE, Leinninger GM (2017) Lateral hypothalamic neurotensin neurons orchestrate dual weight loss behaviors via distinct mechanisms. Cell Rep 21(11):3116–3128
Yousefvand S, Hamidi F (2020) Role of paraventricular nucleus in regulation of feeding behaviour and the design of intranuclear neuronal pathway communications. Int J Pept Res Ther 26(3):1231–1242
Yousefvand S, Hamidi F (2021) The role of ventromedial hypothalamus receptors in the central regulation of food intake. Int J Pept Res Ther 27(1):689–702
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2018a) Effects of insulin and somatostatin on water intake in neonatal chickens. Iran J Physiol Pharmacol 2(3):165–158
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2018b) Hypophagic effects of insulin are mediated via NPY1/NPY2 receptors in broiler cockerels. Can J Physiol Pharmacol 96(12):1301–1307
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2019) Interaction of neuropeptide Y receptors (NPY1, NPY2 and NPY5) with somatostatin on somatostatin-induced feeding behaviour in neonatal chicken. Br Poult Sci 60(1):71–78
Yousefvand S, Hamidi F, Zendehdel M, Parham A (2020) Survey the effect of insulin on modulating feed intake via NPY receptors in 5-day-old chickens. Int J Pept Res Ther 26(1):467–476
Zendehdel M, Hassanpour S (2014) Central regulation of food intake in mammals and birds: a review. Neurotransmitter 1:1–7
Zendehdel M, Hassanpour S (2014b) Ghrelin-induced hypophagia is mediated by the β 2 adrenergic receptor in chicken. J Physiol Sci 64(5):383–391
Zendehdel M, Hamidi F, Babapour V, Mokhtarpouriani K, Fard RM (2012) The effect of melanocortin (Mc3 and Mc4) antagonists on serotonin-induced food and water intake of broiler cockerels. J Vet Sci 13(3):229
Zendehdel M, Mokhtarpouriani K, Babapour V, Pourrahimi M, Hamidi F (2013a) The role of 5-HT2A and 5-HT2C receptors on harmalineinduced eating behavior in 24-h food-deprived broiler cockerels. Iran J Vet Res 14(2):94–99
Zendehdel M, Mokhtarpouriani K, Hamidi F, Montazeri R (2013b) Intracerebroventricular injection of ghrelin produces hypophagia through central serotonergic mechanisms in chicken. Vet Res Commun 37(1):37–41
Zendehdel M, Hamidi F, Hassanpour S (2015) The effect of histaminergic system on nociceptin/orphanin FQ induced food intake in chicken. Int J Pept Res Ther 21(2):179–186
Zendehdel M, Parvizi Z, Hassanpour S, Baghbanzadeh A, Hamidi F (2017) Interaction between nociceptin/orphanin FQ and adrenergic system on food intake in neonatal chicken. Int J Pept Res Ther 23(1):155–161
Zendehdel M, Ebrahimi-Yeganeh A, Hassanpour S, Koohi MK (2019) Interaction of the dopaminergic and nociceptin/orphanin FQ on central feed intake regulation in chicken. Br Poult Sci 60(3):317–322
Zendehdel M, Hassanpour S, Movahedi N (2020) Central and peripheral methylamine-induced hypophagia is mediated via nitric oxide and TAAR1 in neonatal layer-type chicken. Neurosci Lett 739:135408
Zhang L, Hernandez-Sanchez D, Herzog H (2019) Regulation of feeding-related behaviors by arcuate neuropeptide Y neurons. Endocrinology 160(6):1411–1420
Zhu Y, Yamanaka A, Kunii K, Tsujino N, Goto K, Sakurai T (2002) Orexin-mediated feeding behavior involves both leptin-sensitive and-insensitive pathways. Physiol Behav 77(2–3):251–257
Acknowledgements
The authors thank the Ferdowsi University of Mashhad for their cooperation and support of this article.
Funding
This review article did not receive any specific grant from funding agencies in the public, commercial, and not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
In this review article, no laboratory work has been done on animals.
Research Involving Human Participants and/or Animals
No humans/or animals participated in this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousefvand, S., Hamidi, F. Role of Lateral Hypothalamus Area in the Central Regulation of Feeding. Int J Pept Res Ther 28, 83 (2022). https://doi.org/10.1007/s10989-022-10391-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-022-10391-4