Abstract
To select specific binding peptides for imaging and detection of human ovarian cancer. The phage 12-mer peptide library was used to select specific phage clones to ovarian cancer cells. After four rounds of biopanning, the binding specificity of randomly selected phage clones to ovarian cancer cells was determined by enzyme-linked immunosorbent assay (ELISA). DNA sequencing and homology analysis were performed on specifically bound phages. The binding ability of the selected peptides to SKOV3 cells was confirmed by fluorescence microscopy and flow cytometry. After four rounds of optimized biological panning, phage recovery was 34-fold higher than that of the first round, and the specific phage clones bound to SKOV3 cells were significantly enriched. A total of 32 positive phage clones were preliminarily identified by ELISA from 54 randomly selected clones, and the positive rate was 59.3%. S36 was identified as the clone with best affinity to SKOV3 cells via fluorescence microscopy and flow cytometry. A representative clone of OSP2, S36 is expected to be an effective probe for diagnosis and treatment of ovarian cancer.
Similar content being viewed by others
Introduction
The incidence and mortality of ovarian cancer rank first among cancers of the female reproductive system, and the trend of younger generation is obvious, posing a serious threat to women’s health (Siegel et al. 2019). Survival rate of ovarian cancer mainly depends on early detection, but early symptoms of ovarian cancer are not obvious, most patients are diagnosed at stage III or IV, of which the 5-year cause-specific survival were 42% and 26%, respectively. However, for those patients diagnosed at stage I and II, the 5-year survival rate can be as high as 89% and 71% (Torre et al. 2018). Serum carbohydrate antigen 125 and human epididymal epithelium secreted protein 4, as well as ovarian cancer risk prediction model which based on the levels of these two serum biomarkers and the menopausal status of patients, are of great significance for assessing the risk of ovarian cancer (Chudecka-Glaz et al. 2016; Dayyani et al. 2016; Dochez et al. 2019; Romagnolo et al. 2016). However, these indicators are not specific in the early diagnosis of ovarian cancer. Thus, improved detection of ovarian cancer is needed to enhance prognosis for patients.
Phage display technology is a unique gene recombination expression method described by Smith (1985). Proteins or peptides with high affinity and selectivity to target molecules can be obtained through insertion of the gene encoding the corresponding polypeptide into the phage genome (Parmley and Smith 1988). Ligands can be identified with no prior information concerning antibody specificity (Cwirla et al. 1990). Phage display technology has not only been successfully applied in various biomedical fields such as molecular imaging (Haque et al. 2019), vaccine development (Toledo-Machado et al. 2015), antibody engineering (Muchima et al. 2018) and nanotechnology (Hofmeister et al. 2015), but also shown its unique advantages in the detection of tumor-specific target molecules and targeted therapies. Studies have shown that peptide probes with high specificity and affinity have been identified for cervical cancer (Xiao et al. 2019), breast cancer (Liu et al. 2019), pancreatic cancer (Asar et al. 2020), colon cancer (Kwak et al. 2020a) and esophageal cancer (Ma et al. 2015).
In this study, we have tried to find out novel peptides targeting SKOV3 cells from a liner 12-mer phage display peptide library, indicating its potential as an useful molecule to imaging detection and targeting therapy for ovarian cancer.
Materials and Methods
Cell lines and Cell Culture
SKOV3 (human ovarian cancer cell), Hela (human cervical cancer), AN3CA (human endometrial cancer cell), HEK293 (human embryonic kidney cell) are all from Department of Obstetrics and Gynecology Laboratory, MCF7 (human breast cancer cell) and MHCC97-H (human highly metastatic liver cancer cell) were donated from the Department of Physiology and Diseases Laboratory. All cell lines were maintained in RPMI-1640 medium supplemented with 10% [v/v] heat-inactivated fetal bovine serum (FBS), 100 U penicillin/ml and 100 mg streptomycin/ml at 37 °C in a humidified atmosphere with 5% CO2.
Phage Display Biopanning
Ph.D.-12 liner peptide library kit was purchased from New England Biolabs (NEB, Beverly, USA). SKOV3 and HEK293 cells were used as positive target cells and negative absorber cells, respectively. Cells (5 × 105/ml) were seeded on polylysine coated plates, and cultured overnight with RPMI-1640 medium containing 10% FBS to 80–90% confluence. HEK293 and SKOV3 cells were cultured with serum-free medium for 1 h, blocked with 3% bovine serum albumin (BSA) for 2 h at 37 °C and washed three times with TBST (TBS + 0.1% Tween-20 [v/v]) buffer. Approximately 1 × 1011 pfu phages were incubated with HEK293 cells at 37 °C for 1 h. After incubation, the supernatant containing unbound phages was incubated with the blocked SKOV3 cells at 37 °C for 2 h. The cells were washed four times with 0.1% TBST to remove unbound and nonspecific phages, bound phages were eluted with elution buffer (260 μl, 0.2 M Glycine–HCl (pH 2.2)) for 20 min and neutralized with 40 μl of 1 M Tris–HCl (pH 9.1). The eluted phages were titered and amplified as described in the manufacturer’s protocol. These amplified phages were subjected to the next round of biopanning. To minimize non-specific binding, HEK293 cells were chosen for biopanning against peptide library before SKOV3 cells in each round.
Enzyme-Linked Immunosorbent Assay
After the fourth round of biopanning, 54 phage clones were randomly picked out from titered phage plaques for ELISA. SKOV3 and HEK293 cells were seeded into 96-well plates (4 × 104 cells/well) overnight and then fixed with 4% paraformaldehyde for 20 min at room temperature. 3% H2O2 (100 μl/well) was added, and the plates were placed at room temperature for 30 min to inhibit the activity of endogenous peroxidase. Then cells were blocked with blocking buffer (TBST containing 3% BSA, 250 μl/well) at 37 °C for 2 h. The selected phages (1 × 1010 pfu/well) were added to SKOV3 and HEK293 cells and incubated at 37 °C for 1.5 h. After that, the cells were washed three times with TBST and cultured with HRP-conjugated anti-M13 antibody (diluted at 1:10,000 in 3% BSA, Sino Biological, Beijing, China) for 1 h. Tetramethylbenzidine (100 μl/well, Solarbio Technology Co., Ltd.) were added to the cells and incubated at 37 °C in the dark for 20 min. The incubation was stopped by adding 100 μl/well stop solution. Finally, the 96-well plates were measured at 450 nm using an ELISA reader (Bio-Tek ELX800, USA). Irrelevant phage clone (IRP, an amplified phage randomly selected from the original phage peptide library) and PBS were used as control groups. The relative binding abilities were calculated by ODSKOV3/ODHEK293, the ratio of absorbance over 2.1 was identified as positive clone.
DNA Sequencing of the Positive Phage Clones
The selected positive phage clones were extracted DNA for the sequencing analysis. Firstly, Escherichia coli ER2738 were inoculated in 10 mL LB + Tet, shaken vigorously overnight and diluted to 20 ml LB at the next day. Then, 10 μl monoclonal phage was added to ER2738 and the mixture was shaken at 37 °C for 4.5 h, and the supernatant was harvested at 12,000×g for 10 min. 200 μl of PEG/NaCl was added to the supernatant to precipitate the phages. The precipitate was suspended in iodide buffer, and followed by ethanol precipitation at room temperature for 10 min. The ssDNA was recovered and dissolved in TE buffer. DNA sequencing of the selected phages was carried out by Sangon Biotech (ShangHai, China). The primer used for sequencing was -96gIII 5´-HO CCC TCA TAG TTA GCG TAA CG-3´. The encoded peptide sequence was deduced from the DNA sequence. Homologous analysis were performed according to the BLAST (https://blast.ncbi.nlm.nih.gov/Blast.).
Immunofluorescence Staining of Positive Phage Clones
SKOV3, Hela, AN3CA, MCF7, MHCC97-H and HEK293 cells (4 × 104/well) were cultured on coverslips overnight and then fixed with 4% paraformaldehyde for 30 min at room temperature. Followed by washing with PBS three times and blocking with 1% BSA for 30 min at 37 °C, 1 × 1011 pfu representative clone of positive phage and IRP were added and incubated with the cells at 37 °C for 2 h. Cells were washed with PBS and incubated with anti-M13 antibody (Abcam, USA) at a dilution of 1:200 at 4 °C overnight. Subsequently, cells were washed with PBST and FITC Conjugated AffiniPure Goat Anti-rabbit IgG (working dilution of 1:100, Boster Biological Technology Co., Ltd., China) were added and incubated at 37 °C for 2 h. After washing three times with PBS, DAPI-Staining-solution (Boster Biological Technology Co., Ltd., China) was used to stain the nucleus. The cells were finally observed using an inverted microscope (SP8 STED 3X, Leica, Germany). IRP and PBS were used as control groups. The relative intensity of green fluorescence signal was calculated using Image J.
Flow Cytometry
SKOV3 and HEK293 cells (1 × 106 cells/ml) were fixed with 4% polyformaldehyde at room temperature for 20 min and blocked with 1% BSA for 30 min. 1 × 1010 pfu phages (S36 and IRP) were added and incubated at 37 °C for 1 h. After washing three times with PBS, M13 antibody (1:200, Santa Cruz Biotechnology, Inc., USA) was added for incubation at 37 °C for 40 min. Cells were washed with PBS and incubated with second antibodies FITC Conjugated AffiniPure Goat Anti-mouse IgG (1:100, Boster Biological Technology Co., Ltd., China) at 37 °C for 40 min. After washing three times with PBS, the ability of phages to bind with the cells was detected by flow cytometry (EPICS XL/XL-MCL, Beckman Coulter, USA).
Localization Analysis of S36 to Cancer Cells
AN3CA, Hela, MCF7, MHCC97-H, HEK293 and SKOV3 cells (4 × 104/well) were incubated with the Dil membrane probe (Beyotime, China) at 37 °C for 15 min, washed three times with PBS, blocked with 1% BSA for 30 min. 1 × 1010 pfu phages were incubated with cancer cells for 1 h at 37 °C. After washing three times with PBS, the cells were incubated with anti-M13 antibody (Abcam, USA) at 4 °C overnight. The second antibodies FITC-Goat Anti-rabbit IgG were added and incubated at 37 °C for 2 h. DAPI was used to stain the nucleus, and the slides were observed using laser confocal microscope (FV1000, Olympus, Japan).
Statistical Analysis
Each experiment was repeated at least 3 times. Statistical analysis was performed using SPSS 20 (IBM, Armonk, NY, USA). All data are expressed as the mean ± S.D. Independent-Samples t-test was used to analyze the differences between the means. *P < 0.05 was considered statistically significant.
Results
Screening of SKOV3 Cells Specifically Binding Phage Clones
To screen phages that bound specifically to human ovarian cancer cells, four rounds of in vitro subtraction screening were performed. As shown in Table 1, after four rounds of biopanning, the recovery rate was 34 times higher than that of the first round (from 3.7 × 10−5 to1.26 × 10−3). The results revealed that phages specifically binding to SKOV3 cells were significantly enriched.
Binding Activity of Phage Clones by ELISA
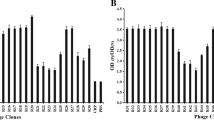
After the fourth round of screening, 54 phage clones were randomly selected, and verified the specific binding ability to SKOV3 cells by ELISA. As shown in Fig. 1, 32 phage clones were identified as positive clones with high binding ability to SKOV3 cells compared to other clones, IRP and PBS controls. The positive rate was 59.3%.
Identification of the phages that bound specifically to the human ovarian cancer cells. Phage clones binding to SKOV3 were detected by incubation with HRP-conjugated anti-M13 phage antibody. a ELISA results of phage clones S1–S28. b ELISA results of phage clones S29-S54, IRP and PBS. The relative binding ability were analyzed by the ratio of absorbance of SKOV3 cells and absorbance of HEK293 cells. IRP and PBS were used as negative controls. Error bars represent the standard deviation of three replicates
Sequencing of the Positive Phages
A total of 32 positive phage clones were sequenced and homologous analysis was performed. As shown in Table 2, phages with same sequence were classified, and twelve peptide sequences were obtained and named OSP1 to OSP12. The OSP1, OSP2, OSP3, OSP4, OSP5, and OSP6 emerged six, five, five, four, three, three times respectively, and the rest of the sequences all emerged once. At the same time, the corresponding phage clones S8, S36, S35, S10, S2, S3 of OSP1 to OSP6 have been further studied because of high binding ratio. Homologous analysis was identified by BLAST between the peptides and the known proteins. However, no complete homologous protein was found, and the peptide with the lowest E value was OSP2 (Table 3).
Affinity Analysis of Positive Phage Clones
Based on the results of ELISA and DNA sequencing, the positive clones S2, S3, S8, S10, S35, and S36 were used for further identification. Cell immunofluorescence assay was performed to detect the specific binding of the six phage clones to SKOV3 cells. As shown in Fig. 2a, the six phage clones all bound SKOV3 cells, whereas only background staining was observed in IRP and PBS groups and the phage S36 bound preferably to SKOV3 cells than other phage clones. Relative intensity of green fluorescence signal was calculated by Image J software. The binding ability of the six phage clones was significantly different to SKOV3 cells, and phage S36 showed strongest binding to SKOV3 cells. (Fig. 2b). S36 was analyzed by flow cytometry as shown in Fig. 2c. S36 has specific binding activity to SKOV3 cells than IRP, and the mean binding rate of S36 and IRP showed no difference to HEK293 cells.
Affinity analysis of positive phage clones. a Immunofluorescence staining of SKOV3 cells with phage clones S2, S3, S8, S10, S35, S36. The cell nucleus were shown in blue (DAPI) and the phages were shown in green (FITC). Scale bar = 25 μm. b Fluorescence signal intensity analysis. The six phage clones had a significantly higher affinity for SKOV3 than IRP and PBS controls. IOD/Area were calculated as the average green fluorescence intensity. Values were shown as the mean ± standard deviation. ***P < 0.001. c Flow cytometry analysis of S36 binding to SKOV3 cells (Color figure online)
To further evaluate the specificity, S36 was selected to bind to other cancer cells, such as cervical (Hela), endometrial (AN3CA), breast (MCF7) and highly metastatic liver (MHCC97-H) cancers. Figure 3 shows that S36 specifically bound to SKOV3 cells, but showed low affinity to Hela, AN3CA, MCF7 and MHCC97-H cells. These results suggested that S36 was the best clone with specific affinity to SKOV3 cells.
Localization Analysis of S36 Clone to Cancer Cells
Localization of S36 clone was further analyzed on a range of cancer cell lines by specific membrane staining using Dil. As shown in Fig. 4, FITC-labeled S36 clone showed specific fluorescence on the cell membrane of SKOV3, there was no significant green fluorescence in other cancer cells including AN3CA, Hela, MCF7, MHCC97-H and HEK293 cells. At the same time, the FITC-labeled IRP bound with low affinity to SKOV3 cells (Control). The results revealed that S36 showed specific staining on SKOV3 cells, but not on other cell lines.
Discussion
The prognosis of patients with ovarian cancer is closely related to the stage of the disease, it is necessary to find new biomarkers to improve diagnosis, treatment and prognosis of ovarian cancer. Phage display technology is a simple and effective tool for screening peptides. The premade libraries include linear heptapeptide (Ph.D.-7) and dodecapeptide (Ph.D.-12) libraries, as well as a loop-constrained heptapeptide library (Dmitrieva et al. 2020). M13 phage is the most widely used vector which consists of a circular single-stranded DNA (ssDNA) genome covered by the major coat protein pVIII and minor coat proteins pIII, pVI, pVII and pIX. These five coat proteins are all used to display foreign peptides on the surface of M13 phage, but the minor protein pIII is the most commonly used (Tonelli et al. 2012). The inserted exogenous peptides or proteins do not affect the phage's structure and infectivity, and can maintain relatively independent spatial structure and biological activity (Devlin et al. 1990). In this study, we identified a peptide, which could be effectively recognized ovarian cancer SKOV3 cells through a liner Ph.D.-12 peptide library.Phage display peptide screening methods mainly include in vivo (Pasqualini and Ruoslahti 1996) and in vitro (Zhang et al. 2007) screening. In vivo phage screening refers to peptide library screening in live animals. The peptide library is injected intravenously into the animal, and the phages can selectively target different tissues to obtain tissue-specific peptides (Pasqualini and Ruoslahti 1996). In vivo screening also has some limitations. Because of different species, peptides isolated from animal models using this method may not be transformed into humans (Wu et al. 2016). In vitro biopanning (also often called whole-cell panning) is used to isolate peptides linked to specific cell targets. Usually, a given cell type has hundreds or thousands of different receptors, each could theoretically acquire a specific ligand from the library, leading to a higher non-specific binding between phages and cells, which makes the unsatisfactory screening effect of tumor cells. Obtained peptides that specifically bind to cells depend both on the strength and stringency of the panning procedure. In this study, to minimize non-specific binding, HEK293 cells were firstly chosen for biopanning against peptide library before SKOV3 cells in each round. In addition, phages with high affinity for their targets were isolated by changing the washing conditions (increasing washing times and Tween-20 concentration).
Recently, a few peptides targeting to ovarian cancer cells have been successfully screened. Zhang et al. also used phage display technology to obtain short peptides that specifically bound to ovarian cancer SKOV3 cells from a random 12 peptide library. Through 5 rounds of biopanning, 10 sequences were obtained from 20 randomly selected clones. Z1 had the highest specificity for binding to ovarian cancer SKOV3 cells (Zhang et al. 2011). According to the protocol of phage peptide library kit, 3–4 rounds of biopanning are recommended. Increasing the panning rounds will enrich non-specifically bound phages. In our study, after 4 rounds of screening, phage recovery rate increased 34 times. A total of 32 positive phages were confirmed by ELISA and 12 sequencing results were obtained. Immunofluorescence and flow cytometry observed S36 was the best clone with highest binding ability to ovarian cancer SKOV3 cells. The peptide sequences are different from the results of Zhang et al. that may be related to stringency of the screening procedures.
Due to the presence of specific epitopes or antigens on the surface of ovarian cancer cells, phage S36 specifically binds to ovarian cancer SKOV3 cells. Through DNA sequencing and homology analysis, we found that S36 (METRPVAPHEFR) is highest homology with antibody heavy chain junction region. Immunoglobulin is a Y-shaped structural protein consisting of two identical light chains and two identical heavy chains. Specificity and affinity of antibodies are determined by an antigen-binding fragment (Fab) (Nagano and Tsutsumi 2021). Cell surface epitope or antigen specifically bound to the phage clone S36 also bind to the heavy chain of immunoglobulin, which may be of great help for further research on the binding sites of ovarian cancer-specific antibodies. High molecular weight and poor tissue penetration of antibodies reduce its aggregation in the tumor site, which may be the reason for the unsatisfactory effect of tumor treatment. Compared with antibodies, peptides have some advantages of small molecular weight, low immunogenicity, high affinity, strong tissue penetration and easy synthesis. Phage clone S36 is expected to be an effective probe combined with appropriate fluorescent markers, liposomes or anti-tumor drugs for early tumor imaging diagnosis and targeted therapy (Li et al. 2020; Yeh et al. 2016), which has important practical significance for solving the problems faced by the prevention and treatment of ovarian cancer.
Further studies needed to synthesize peptides according to the OSP2 sequence, and verified the specificity and sensitivity in ovarian cancer cells, clinical tissue samples and animal models.
References
Asar MC, Franco A, Soendergaard M (2020) Phage display selection, identification, and characterization of novel pancreatic cancer targeting peptides. Biomolecules. https://doi.org/10.3390/biom10050714
Chudecka-Glaz A, Cymbaluk-Ploska A, Luterek-Puszynska K, Menkiszak J (2016) Diagnostic usefulness of the Risk of Ovarian Malignancy Algorithm using the electrochemiluminescence immunoassay for HE4 and the chemiluminescence microparticle immunoassay for CA125. Oncol Lett 12:3101–3114. https://doi.org/10.3892/ol.2016.5058
Cwirla SE, Peters EA, Barrett RW, Dower WJ (1990) Peptides on phage: a vast library of peptides for identifying ligands. Proc Natl Acad Sci USA 87:6378–6382. https://doi.org/10.1073/pnas.87.16.6378
Dayyani F, Uhlig S, Colson B, Simon K, Rolny V, Morgenstern D, Schlumbrecht M (2016) Diagnostic performance of risk of ovarian malignancy algorithm against CA125 and HE4 in connection with ovarian cancer: a meta-analysis. Int J Gynecol Cancer 26:1586–1593. https://doi.org/10.1097/IGC.0000000000000804
Devlin JJ, Panganiban LC, Devlin PE (1990) Random peptide libraries: a source of specific protein binding molecules. Science 249:404–406. https://doi.org/10.1126/science.2143033
Dmitrieva MD, Voitova AA, Dymova MA, Richter VA, Kuligina EV (2020) Tumor-targeting peptides search strategy for the delivery of therapeutic and diagnostic molecules to tumor cells. Int J Mol Sci. https://doi.org/10.3390/ijms22010314
Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G (2019) Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res 12:28. https://doi.org/10.1186/s13048-019-0503-7
Haque ME et al (2019) A phage display-identified peptide selectively binds to kidney injury molecule-1 (KIM-1) and detects KIM-1-overexpressing tumors in vivo. Cancer Res Treat 51:861–875. https://doi.org/10.4143/crt.2018.214
Hofmeister LH, Lee SH, Norlander AE, Montaniel KR, Chen W, Harrison DG, Sung HJ (2015) Phage-display-guided nanocarrier targeting to atheroprone vasculature. ACS Nano 9:4435–4446. https://doi.org/10.1021/acsnano.5b01048
Kwak MH et al (2020) A dodecapeptide selected by phage display as a potential theranostic probe for colon cancers. Transl Oncol 13:100798. https://doi.org/10.1016/j.tranon.2020.100798
Li C et al (2020) Application of Phage-displayed peptides in tumor imaging diagnosis and targeting therapy. Int J Pept Res Ther. https://doi.org/10.1007/s10989-020-10108-5
Liu T, Li B, Jiang Y, Zheng C, Zhang L, Wang Y (2019) Screening and identification of novel specific markers of breast cancer stem cells. Oncol Lett 18:2262–2269. https://doi.org/10.3892/ol.2019.10535
Ma C et al (2015) Screening of a specific peptide binding to esophageal squamous carcinoma cells from phage displayed peptide library. Mol Cell Probes 29:182–189. https://doi.org/10.1016/j.mcp.2015.04.001
Muchima K et al (2018) Development of sugar chain-binding single-chain variable fragment antibody to adult T-cell leukemia cells using glyco-nanotechnology and phage display method. J Biochem 163:281–291. https://doi.org/10.1093/jb/mvy005
Nagano K, Tsutsumi Y (2021) Phage display technology as a powerful platform for antibody drug discovery. Viruses 13:178. https://doi.org/10.3390/v13020178
Parmley SF, Smith GP (1988) Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 73:305–318. https://doi.org/10.1016/0378-1119(88)90495-7
Pasqualini R, Ruoslahti E (1996) Organ targeting in vivo using phage display peptide libraries. Nature 380:364–366. https://doi.org/10.1038/380364a0
Romagnolo C et al (2016) HE4, CA125 and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools for ovarian cancer in patients with a pelvic mass: an Italian multicenter study. Gynecol Oncol 141:303–311. https://doi.org/10.1016/j.ygyno.2016.01.016
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315–1317. https://doi.org/10.1126/science.4001944
Toledo-Machado CM et al (2015) Use of Phage Display technology in development of canine visceral leishmaniasis vaccine using synthetic peptide trapped in sphingomyelin/cholesterol liposomes. Parasit Vectors 8:133. https://doi.org/10.1186/s13071-015-0747-z
Tonelli RR, Colli W, Alves MJ (2012) Selection of binding targets in parasites using phage-display and aptamer libraries in vivo and in vitro. Front Immunol 3:419. https://doi.org/10.3389/fimmu.2012.00419
Torre LA et al (2018) Ovarian cancer statistics, 2018. CA Cancer J Clin 68:284–296. https://doi.org/10.3322/caac.21456
Wu CH, Liu IJ, Lu RM, Wu HC (2016) Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci 23:8. https://doi.org/10.1186/s12929-016-0223-x
Xiao L et al (2019) Development of a novel drug targeting delivery system for cervical cancer therapy. Nanotechnology 30:75604. https://doi.org/10.1088/1361-6528/aaf3f8
Yeh CY, Hsiao JK, Wang YP, Lan CH, Wu HC (2016) Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials 99:1–15. https://doi.org/10.1016/j.biomaterials.2016.05.015
Zhang Y et al (2007) Panning and identification of a colon tumor binding peptide from a phage display peptide library. J Biomol Screen 12:429–435. https://doi.org/10.1177/1087057106299164
Zhang L et al (2011) In vitro screening of ovarian tumor specific peptides from a phage display peptide library. Biotechnol Lett 33:1729–1735. https://doi.org/10.1007/s10529-011-0634-4
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number 81972440) and the First Affiliated Hospital of The Fourth Military Medical University (Grant Number XJZT18MJ54).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declared that there are no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, S., Li, C., Gao, Y. et al. Screening and Identification of a Specific Binding Peptide to Ovarian Cancer Cells from a Phage-Displayed Peptide Library. Int J Pept Res Ther 27, 1741–1749 (2021). https://doi.org/10.1007/s10989-021-10206-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-021-10206-y