Abstract
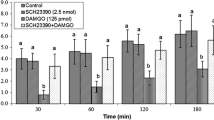
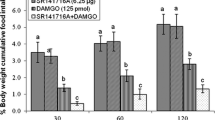
The present study was designed to examine the role of opioidergic and glutamatergic systems on feeding behavior in neonatal meat-type chicken. In experiment 1, FD3 neonatal broilers ICV injected with (A) saline, (B) DAMGO (µ-opioid receptor agonist, 125 pmol), (C) MK-801 (NMDA glutamate receptors antagonist, 15 nmol) and (D) combination of DAMGO plus MK-801. Experiments 2–5 were similar to experiment 1, except FD3 chicks ICV injected with CNQX (AMPA glutamate receptors antagonist, 390 nmol), AIDA (mGLU1 receptors antagonist, 2 nmol), LY341495 (mGLU2 receptors antagonist, 150 nmol) and UBP1112 (mGLU3 receptors antagonist, 2 nmol) instead of MK-801, respectively. In experiments 6–10, FD3 chicks ICV injected as the same as procedure to the experiments 1–5, except to inject with DPDPE (δ-opioid receptor agonist, 40 nmol) instead of the DAMGO. The experiments 11–15 were similar to the experiments 1–5, except neonatal broilers ICV injected with U-50488H (κ-opioid receptor agonist, 30 nmol) instead of DAMGO. Then the cumulative food intake measured until 120 min post injection. According to the results, ICV injection of DAMGO, significantly decreased food intake (P < 0.05) while DPDPE and U-50488H increased feeding behavior compared to the control group (P < 0.05). Co-injection of the DAMGO + MK-801 and DAMGO + AIDA, significantly decreased DAMGO-induced hypophagia in neonatal chicks (P < 0.05). Also, co-injection of the DPDPE + CNQX significantly amplified DPDPE induced feeding behavior (P < 0.05). These results suggested interconnection between central opioidergic and glutamatergic systems on feeding behavior mediates via µ- and δ-opioid receptor with NMDA, AMPA and mGLU1 receptors in FD3 neonatal broilers. These findings may shed light on the circuitry underlying interconnection between central opioidergic and glutamatergic systems on feeding behavior.















Similar content being viewed by others
References
Alimohammadi S, Zendehdel M, Babapour V (2015) Modulation of opioid-induced feeding behavior by endogenous nitric oxide in neonatal layer-type chicks. Vet Res Commun 39:105–113
Baghbanzadeh A, Babapour V (2007) Glutamate ionotropic and metabotropic receptors affect feed intake in broiler cockerels. J Vet Res 62(4):125–129
Beckerman MA, Glass MJ (2011) Ultrastructural relationship between the AMPA-GluR2 receptor subunit and the mu-opioid receptor in the mouse central nucleus of the amygdala. Exp Neurol 227(1):149–158
Blevins JE, Stanley BG, Reidelberger RD (2002) DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav 71:277–282
Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ (2013) Food Restriction Increases Glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci 33(34):13861–13872
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2004) Feeding responses to µ-, δ- and κ-opioid receptor agonists in the meat-type chick. Pharmacol Biochem Behav 78: 707–710
Bungo T, Kawamura K, Izumi T, Dodo K, Ueda H (2005) Effects of various µ-, δ- and κ-opioid ligands on food intake in the meat-type chick. Physiol Behav 85:519–523
Charles JR, Duva MA, Ramirez GJ, Lara RL, Yang CR, Stanley BG (2014) Activation of lateral hypothalamic mGlu1 and mGlu5 receptors elicits feeding in rats. Neuropharmacology 79:59–65
Da Silva AA, Marino-Neto J, MA P (2003) Feeding induced by microinjections of NMDA and AMPA–kainite receptor antagonists into ventral striatal and ventral pallidal areas of the pigeon. Brain Res 966:76–83
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Denbow DM (1994) Peripheral regulation of food intake in poultry. J Nutr 124:1349S–1354S
Farahmandfar M, Karimian SM, Zarrindast MR, Kadivar M, Afrouzi H, Naghdi N (2011) Morphine sensitization increases the extracellular level of glutamate in CA1 of rat hippocampus via µ-opioid receptor. Neurosci Lett 494: 130–134
Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y (2012) Current research on opioid receptor function. Curr Drug Target 13(2):230–246
Filizola M, Devi LA (2013) Grand opening of structure-guided design for novel opioids. Trends Pharmacol Sci 34(1):6–12
Furuse M (2002) Central regulation of food intake in the neonatal chick. Anim Sci J 73:83–94
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Garzón J, Rodríguez-Muñoz M, Sánchez-Blázquez P (2012) Direct association of mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance. Curr Drug Abuse Rev 5:199–226
Glass MJ, Lane DA, Colago EE, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM (2008) Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol 210:750–761
Guo M, Xu NJ, Li YT, Yang JY, Wu CF, Pei G (2005) Morphine modulates glutamate release in the hippocampal CA1 area in mice. Neurosci Lett 381: 12–15
Guo Y, Wang HL, Xiang XH, Zhao Y (2009) The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neurosci Biobehav Rev 33:864–873
Hao Y, Yang JY, Wu CF, Wu MF (2007) Pseudoginsenoside-F11 decreases morphine-induced behavioral sensitization and extracellular glutamate levels in the medial prefrontal cortex in mice. Pharmacol Biochem Behav 86:660–666
Hettes SR, GonzagaWJ, Heyming TW, Nguyen JK, Perez S, Stanley BG (2010) Stimulation of lateral hypothalamic AMPA receptors may induce feeding in rats. Brain Res 1346:112–120
Kamali M, Sahraei H, Khosravi M, Hassanpour S, Yaribeygi H (2016) Asymmetric involvement of central and the peripheral NMDA glutamate receptors in the expression of withdrawal syndrome in morphine-dependent mice. Physiol Pharmacol 19: 274–284
Kaneko K, Yoshikawa M, Ohinata K (2012) Novel orexigenic pathway prostaglandin D2-NPY system-involvement in orally active orexigenic δ opioid peptide. Neuropeptides 46:353–357
Ladepeche L, Yang L, Bouchet D, Groc L (2013) Regulation of dopamine D1 receptor dynamics within the postsynaptic density of hippocampal glutamate synapses. PLoS ONE 8(9):e74512
Le Merrer J, Becker JAJ, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Lee CWS, Ho IK (2013) Pharmacological profiles of oligomerized μ-opioid receptors. Cells 2, 689–714. doi:10.3390/cells2040689
Liu XJ, Salter MW (2010) Glutamate receptor phosphorylation and trafficking in pain plasticity in spinal cord dorsal horn. Eur J Neurosci 32:278–289
McFadden KL, Cornier MA, Tregellas JR (2014) The role of alpha-7 nicotinic receptors in food intake behaviors. Frontiers In Psychol 5(553):1–7
Mena JD, Selleck RA, Baldo BA (2013) Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci 33(47):18540–18552
Minowa S, Ishihara S, Tsuchiya S, Horie S, Watanabe K, Murayama T (2003) Involvement of glutamate and c-amino-butyric acid receptor systems on gastric acid secretion induced by activation of κ-opioid receptors in the central nervous system in rats. Br J Pharmacol 138:1049–1058
Mitrano DA, Arnold C, Smith Y (2008) Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocainetreated rats. Neurosci 154: 653–666
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J Poult Sci 5(4):301–308
Qi W, Ding D, Salvi RJ (2008) Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res 236:52–60
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Scavone JL, Asan E, Van Bockstaele EJ (2011) Unraveling glutamate-opioid receptor interactions using highresolution electron microscopy: implications for addictionrelated processes. Exp Neurol 229(2):207–213
Schotanus SM, Chergui K (2008) Dopamine D1 receptors and group I metabotropic glutamate receptors contribute to the induction of long-term potentiation in the nucleus accumbens. Neuropharmacol 54: 837–844
Sepehrizadeh Z, Bahrololoumi Shapourabadi M, Ahmadi S, Bozchlou Hashemi S, Zarrindast MR, Sahebgharani M (2008a) Decreased AMPA GluR2, but not GluR3, mRNA expression in rat amygdala and dorsal hippocampus following morphine-induced behavioural sensitization. Clin Exp Pharmacol Physiol 35:1321–1330
Sepehrizadeh Z, Sahebgharani M, Ahmadi S, Shapourabadi MB, Bozchlou Hashemi S, Zarrindast MR (2008b) Morphine-induced behavioral sensitization increased the mRNA expression of NMDA receptor subunits in the rat amygdala. Pharmacol 81:333–343
Shojaei M, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S (2015) Opioid-induced hypophagia is mediated by 5-HT2c receptors in neonatal layer-type chicken. Czech J Anim Sci 60(9):400–410
Steinman JL, Fujikawa DG, Wasterlain CG, Cherkin A, Morley JE (1987) The effects of adrenergic, opioid and pancreatic polypeptidergic compounds on feeding and other behaviors in neonatal leghorn chicks. Peptides 8: 585–592
Taati M, Nayebzadeh H, Zendehdel M (2011) The effects of DLAP5 and glutamate on ghrelin-induced feeding behavior in 3-h food-deprived broiler cockerels. J Physiol Biochem 67:217–223
Taheriyan MR, Baghbanzadeh A, Zendehdel M (2016) Dopamine- induced hypophagia is mediated via NMDA and mGlu1 receptors in chicken. Iranian J. Vet Med 10(3):191–199
Tzschentke TM, Schmidt WJ (2003) Glutamatergic mechanisms in addiction. Mol Psychiatr 8:373–382
Van Tienhoven A, Juhasz LP (1962) The chicken telencephalon, diencephalon and mesencephalon in sterotaxic coordinates. J Comp Neurol 118:185–197
Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, Wu CF, Zhang X, Pei G (2003) Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci 23:4775–4784
Zendehdel M, Baghbanzadeh A, Babapour V, Cheraghi J (2009) The effects of bicuculline and muscimol on glutamate-induced feeding behaviour in broiler cockerels. J Comp Physiol A 195:715–720
Zendehdel M, Ghashghayi E, Hassanpour S, Baghbanzadeh A, Jonaidi H (2016) Interaction between opioidergic and dopaminergic systems on food intake in neonatal layer type chicken. Int J Pept Res Ther 22:83–92
Zendehdel M, Hassanpour S (2014) Ghrelin-induced hypophagia is mediated by the β2 adrenergic receptor in chicken. J Physiol Sci 64:383–391
Zendehdel M, Hassanpour S, Babapour V, Charkhkar Mahdavi M (2015) Interaction between endocannabinoid and opioidergic systems regulates food intake in neonatal chicken. Int J Pept Res Ther 21:289–297
Zendehdel M, Taati M, Jonaidi H, Amini E (2012) The role of central 5-HT (2C) and NMDA receptors on LPS-induced feeding behavior in chickens. J Physiol Sci 62:413–419
Zeni LA, Seidler HB, De Carvalho NA, Freitas CG, Marino-Neto J, Paschoalini MA (2000) Glutamatergic control of food intake in pigeons: effects of central injections of glutamate, NMDA, and AMPA receptor agonists and antagonists. Pharmacol Biochem Behav 65(1):67–74
Acknowledgements
The authors thank the central laboratory (Dr. Rastegar Lab.) of the Faculty of Veterinary Medicine, University of Tehran for cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Participants
All experiments were executed according to the Guide for the Care and Use of Laboratory Animals and were approved by the institutional animal ethics committee.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Torkzaban, M., Zendehdel, M., Babapour, V. et al. Interaction Between Central Opioidergic and Glutamatergic Systems on Food Intake in Neonatal Chicks: Role of NMDA, AMPA and mGLU1 Receptors. Int J Pept Res Ther 24, 157–169 (2018). https://doi.org/10.1007/s10989-017-9601-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-017-9601-9