Abstract
Context
Given the importance of spatial heterogeneity in altering dispersal, interspecific interactions, and population persistence, high rates of habitat homogenisation across the globe are a concern. In river ecosystems, confluences likely act as heterogeneity ‘hotspots’ by creating discontinuities in longitudinal processes and influences that are propagated both up and down stream networks.
Objectives
We predicted the layout of abiotic conditions around confluences would influence the presence and configuration of spatial heterogeneity, and strongly influence spatial patterns in fish abundance and evenness.
Methods
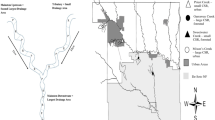
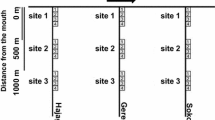
Twelve replicate mainstem and tributary stream combinations in Canterbury, New Zealand, were electrofished to evaluate how the configuration of flow disturbance (i.e. flooding) characteristics around a confluence (i.e. stable mainstem and disturbed tributary versus disturbed mainstem and stable tributary) influenced fish communities.
Results
The configuration of abiotic conditions around confluences, and position of a sampled reach with respect to the confluence, significantly interacted to create configuration-specific patterns in fish abundance and evenness. Fish abundances were particularly high in disturbed tributaries juxtaposed with stable mainstems, suggesting certain confluence configurations are disproportionately ecologically important. Evenness scores differed significantly downstream of confluences, depending on configuration, with highest fish evenness in reaches downstream of confluences between a stable and a disturbed stream.
Conclusions
These results reveal strongly context-dependent spatial patterns in communities and demonstrate the role of spatially transferred influence in river systems. Understanding importance of not just the presence of heterogeneity, but its configuration and context-dependence in the landscape will assist in identifying sites of ecological significance for management and conservation purposes.






Similar content being viewed by others
References
Barton K (2016) MuMIn: multi-model inference. R package version 1.15.6. https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Benda L, Poff NL, Miller D, Dunne T, Reeves G, Pess G, Pollock M (2004) The network dynamics hypothesis: how channel networks structure riverine habitats. Bioscience 54:413–427
Boddy NC, McIntosh AR (2017) Temperature, invaders and patchy habitat interact to limit the distribution of a vulnerable freshwater fish. Austral Ecol 42:456–467
Bosem Baillod A, Tscharntke T, Clough Y, Batáry P (2017) Landscape-scale interactions of spatial and temporal cropland heterogeneity drive biological control of cereal aphids. J Appl Ecol 54:1804–1813
Brewitt KS, Danner EM, Moore JW (2017) Hot eats and cool creeks: juvenile Pacific salmonids use mainstem prey while in thermal refuges. Can J Fish Aquat Sci 74:1588–1602
Brose U, Martinez ND, Williams RJ (2003) Estimating species richness: sensitivity to sample coverage and insensitivity to spatial patterns. Ecology 84:2364–2377
Brown LE, Hannah DM, Milner AM (2007) Vulnerability of alpine stream biodiversity to shrinking glaciers and snowpacks. Glob Change Biol 13:958–966
Campbell Grant EH, Lowe WH, Fagan WF (2007) Living in the branches: population dynamics and ecological processes in dendritic networks. Ecol Lett 10:165–175
Cromsigt JPGM, Prins HHT, Olff H (2009) Habitat heterogeneity as a driver of ungulate diversity and distribution patterns: interaction of body mass and digestive strategy. Div Distrib 15:513–522
Czeglédi I, Sály P, Takács P, Dolezsai A, Nagy SA, Erős T (2015) The scales of variability of stream fish assemblages at tributary confluences. Aquat Sci 78:641–654
Dauwalter DC, Pert EJ (2003) Effect of electrofishing effort on an index of biotic integrity. N Am J Fish Manag 23:1247–1252
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Cam Philos Soc 81:163–182
Fernandes CC, Podos J, Lundberg JG (2004) Amazonian ecology: tributaries enhance the diversity of electric fishes. Science 305:1960–1962
Ficetola GF, De Bernardi F (2005) Supplementation or in situ conservation? Evidence of local adaptation in the Italian agile frog Rana latastei and consequences for the management of populations. Anim Conserv 8:33–40
Flitcroft RL, Burnett KM, Reeves GH, Ganio LM (2012) Do network relationships matter? Comparing network and instream habitat variables to explain densities of juvenile coho salmon (Oncorhynchus kisutch) in mid-coastal Oregon. USA. Aquat. Conserv. 22:288–302
Fox J (2003) Effect Displays in R for Generalised Linear Models. J Stat Softw 8:1–27
Ganio LM, Torgersen CE, Gresswell RE (2005) A geostatistical approach for describing spatial pattern in stream networks. Front Ecol Environ 3:138–144
Garcia XF, Schnauder I, Pusch MT (2012) Complex hydromorphology of meanders can support benthic invertebrate diversity in rivers. Hydrobiologia 685:49–68
Green JC (2003) The precision of sampling grain size percentiles using the Wolman method. Earth Surf Proc Land 28:979–991
Grossman GD, Ratjczak RE, Crawford M, Freeman MC (1998) Assemblage organization in stream fishes: effects of environmental variation and interspecific interactions. Ecol. Monograph 68:396–420
Hitt NP, Angermeier PL (2008) Evidence for fish dispersal from spatial analysis of stream network topology. J. N. Am. Benthol. Soc. 27:304–320
Iwasaki A, Noda T (2018) A framework for quantifying the relationship between intensity and severity of impact of disturbance across types of events and species. Sci. Rep. 8:795
Jackson DA, Peres-Neto P, Olden JD (2001) What controls who is where in freshwater fish communities — the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170
Jellyman PG, Booker DJ, McIntosh AR (2013) Quantifying the direct and indirect effects of flow-related disturbance on stream fish assemblages. Freshw Biol 58:2614–2631
Jones NE, Schmidt BJ (2017) Tributary effects in rivers: interactions of spatial scale, network structure, and landscape characteristics. Can J Fish Aquat Sci 74:503–510
Kennedy CG, Mather ME, Smith JM, Finn JT, Deegan LA (2016) Discontinuities concentrate mobile predators: quantifying organism-environment interactions at a seascape scale. Ecosphere 7:e01226
Kiffney PM, Greene CM, Hall JE, Davies JR (2006) Tributary streams create spatial discontinuities in habitat, biological productivity, and diversity in mainstem rivers. Can J Fish Aquat Sci 63:2518–2530
Lake PS (2000) Disturbance, patchiness, and diversity in streams. J. N. Am. Benthol. Soc. 19:573–592
Leathwick JR, Elith J, Chadderton WL, Rowe D, Hastie T (2008) Dispersal, disturbance and the contrasting biogeographies of New Zealand’s diadromous and non-diadromous fish species. J Biogeogr 35:1481–1497
Magalhães MF, Beja P, Canas C, Collares-Pereira MJ (2002) Functional heterogeneity of dry-season fish refugia across a Mediterranean catchment: the role of habitat and predation. Freshw Biol 47:1919–1934
Matthaei CD, Arbuckle CJ, Townsend CR (2000) Stable surface stones as refugia for invertebrates during disturbance in a New Zealand stream. J N Am Benthol Soc 19:82–93
McCluney KE, Poff NL, Pakiffnlmer MA, Thorp JH, Poole GC, Williams BS, Williams MR, Baron JS (2014) Riverine macrosystems ecology: sensitivity, resistance, and resilience of whole river basins with human alterations. Front Ecol Environ 12:48–58
McDowall RM (2003) Impacts of introduced salmonids on native galaxiids in New Zealand upland streams: a new look at an old problem. Trans Am Fish Soc 132:229–238
McHugh PA, McIntosh AR, Jellyman PG (2010) Dual influences of ecosystem size and disturbance on food chain length in streams. Ecol Lett 13:881–890
McIntosh AR, Crowl TA, Townsend CR (1994) Size-related impacts of introduced brown trout on the distribution of native common river galaxias. N Z J Mar Freshw Res 28:135–144
McIntosh AR, McHugh PA, Dunn N, Goodman JM, Howard SW, Jellyman PG, O’Brien LK, Nyström P, Woodford DJ (2010) The impact of trout on galaxiid fishes in New Zealand. N Z J Ecol 34:195–206
MEA (2005) Millennium Ecosystem Assessment. Ecosystems and human well-being: synthesis. Island Press, Washington DC
Milesi SV, Melo AS, Chen Y (2014) Conditional effects of aquatic insects of small tributaries on mainstream assemblages: position within drainage network matters. Can J Fish Aquat Sci 71:1–9
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Niu SQ, Franczyk MP, Knouft JH (2012) Regional species richness, hydrological characteristics and the local species richness of assemblages of North American stream fishes. Freshw Biol 57:2367–2377
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2017) Vegan: community ecology package. R package version 2.4-3. https://CRAN.R-project.org/package=vegan
Peckarsky BL, McIntosh AR, Horn SC, McHugh K, Booker DJ, Wilcox AC, Brown W, Alvarez M (2014) Characterizing disturbance regimes of mountain streams. Freshw Sci 33:716–730
Peterson E, Ver Hoef JM (2010) A mixed-model moving-average approach to geostatistical modeling in stream networks. Ecology 91:644–651
Pfankuch DJ (1975) Stream reach inventory and channel stability evaluation. United States Department of Agriculture Forest Service, Region 1
Pickett STA, Cadenasso ML (1995) Landscape ecology: spatial heterogeneity in ecological systems. Science 269:331–334
Power ME, Stewart AJ, Matthews WJ (1988) Grazer control of algae in an Ozark mountain stream: effects of short-term exclusion. Ecology 69:1894–1898
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132:652–661
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Foundation for Statistical Computing. https://www.R-project.org/
Rice SP, Greenwood MT, Joyce CB (2001) Tributaries, sediment sources, and the longitudinal organisation of macroinvertebrate fauna along river systems. Can J Fish Aquat Sci 58:824–840
Sarkar D (2008) Lattice: multivariate data visualisation with R. Springer, New York
Scheiner SM, Willig MR (2008) A general theory of ecology. Theor Ecol 1:21–28
Simonson TD, Lyons J (1995) Comparason of catch per unit effort and removal procedures for sampling stream fish assemblages. N Am J Fish Manag 15:419–427
Smith JM, Mather ME (2013) Beaver dams maintain fish biodiversity by increasing habitat heterogeneity throughout a low-gradient stream network. Freshw Biol 58:1523–1538
Townsend CR, Crowl TA (1991) Fragmented population structure in a native New Zealand fish: an effect of introduced brown trout. Oikos 61:347–354
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Woodford DJ, McIntosh AR (2010) Evidence of source-sink metapopulations in a vulnerable native galaxiid fish driven by introduced trout. Ecol Appl 20:967–977
Zeni JO, Casatti L (2014) The influence of habitat homogenisation on the trophic structure of fish fauna in tropical streams. Hydrobiologia 726:259–270
Acknowledgements
We are grateful to Castle Hill, Brooksdale, Flock Hill and Glenthorne Stations and to the Department of Conservation for allowing access to sampling sites. Funding was provided by a subcontract from the New Zealand National Institute for Water and Atmospheric Research under the Freshwater & Estuaries Research Programme 2, Sustainable Water Allocation (2014/15 SCI). We thank Richard White, Brandon Goeller, Sophie Hale, Simon Howard and Tom Moore for field assistance, Linda Morris for Technical support, Jenny Ladley for logistical support, and the University of Canterbury for use of the Cass Field Station. Discussions with Erin Peterson at the Queensland University of Technology, Peter McHugh and Carl Saunders at Utah State University, Phillip Jellyman from the New Zealand National Institute for Water and Atmospheric Research and the Freshwater Ecology Research Group (University of Canterbury) enhanced this manuscript. Procedures were conducted under Animal Ethics permit 2014/28R from the University of Canterbury.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Boddy, N.C., Booker, D.J. & McIntosh, A.R. Confluence configuration of river networks controls spatial patterns in fish communities. Landscape Ecol 34, 187–201 (2019). https://doi.org/10.1007/s10980-018-0763-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-018-0763-4