Abstract
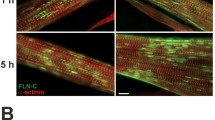
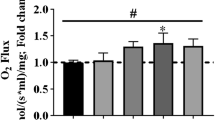
We found that the active tension of C2C12 myotubes that had been subjected to artificial exercise for ~10 days decreased rapidly after termination of the artificial exercise. When differentiated C2C12 myotubes were subjected to continuous 1 Hz artificial exercise for ~10 days, the active tension increased to ~4× compared to that before application of the artificial exercise, as reported previously. On termination of artificial exercise, the active tension decreased rapidly, the level reaching that before application of the artificial exercise within 8 h. Concomitant with the decrease in the active tension, an increase in the amount of ubiquitinated proteins was observed. Real time RT–PCR revealed that the expression of several genes associated with atrophy, namely Smc6, Vegfa, Jarid2, Kitl, Cds2, Inmt, Fasn, Neurl, Topors, and Cul2, were also changed after termination of artificial exercise. These results indicate that termination of artificial exercise induced atrophy-like responses of C2C12 myotubes. Here we found that during the decrease in active tension, the sarcomere structure, especially the thin filament structure, decayed rapidly after termination of artificial exercise. On reapplication of the artificial exercise, the active tension was restored rapidly, within 8 h, concomitant with reformation of the sarcomere structure. These results indicate that disassembly of the sarcomere structure may be one of the reasons for the active tension decrease during disuse muscle atrophy.





Similar content being viewed by others
References
Adams GR, Caiozzo VJ, Baldwin KM (2003) Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 95(6):2185–2201
Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR (1997) Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 273(2 Pt 1):C579–C587
Allen DL, Bandstra ER, Harrison BC, Thorng S, Stodieck LS, Kostenuik PJ, Morony S, Lacey DL, Hammond TG, Leinwand LL, Argraves WS, Bateman TA, Barth JL (2009) Effects of spaceflight on murine skeletal muscle gene expression. J Appl Physiol 106(2):582–595
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294(5547):1704–1708
Booth FW (1982) Effect of limb immobilization on skeletal muscle. J Appl Physiol 52(5):1113–1118
Borisov AB, Dedkov EI, Carlson BM (2001) Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat Rec 264(2):203–218
Enns DL, Raastad T, Ugelstad I, Belcastro AN (2007) Calpain/calpastatin activities and substrate depletion patterns during hindlimb unweighting and reweighting in skeletal muscle. Eur J Appl Physiol 100(4):445–455
Fitts RH, Riley DR, Widrick JJ (2000) Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol 89(2):823–839
Fluck M, Schmutz S, Wittwer M, Hoppeler H, Desplanches D (2005) Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 289(1):R4–R14
Fujita H, Nedachi T, Kanzaki M (2007) Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res 313(9):1853–1865
Fujita H, Shimizu K, Nagamori E (2010) Novel method for measuring active tension generation by C2C12 myotube using UV-crosslinked collagen film. Biotechnol Bioeng 106(3):482–489
Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ (1987) Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 72(4):503–509
Giger JM, Bodell PW, Zeng M, Baldwin KM, Haddad F (2009) Rapid muscle atrophy response to unloading: pretranslational processes involving MHC and actin. J Appl Physiol 107(4):1204–1212
Goll DE, Neti G, Mares SW, Thompson VF (2008) Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86(14 Suppl):E19–E35
Hirano M, Zang L, Oka T, Ito Y, Shimada Y, Nishimura Y, Tanaka T (2006) Novel reciprocal regulation of cAMP signaling and apoptosis by orphan G-protein-coupled receptor GPRC5A gene expression. Biochem Biophys Res Commun 351(1):185–191
Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE (2005) Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288(5):R1288–R1296
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358(13):1327–1335
Midrio M, Danieli-Betto D, Megighian A, Velussi C, Catani C, Carraro U (1992) Slow-to-fast transformation of denervated soleus muscle of the rat, in the presence of an antifibrillatory drug. Pflugers Arch 420(5–6):446–450
Nikawa T, Ishidoh K, Hirasaka K, Ishihara I, Ikemoto M, Kano M, Kominami E, Nonaka I, Ogawa T, Adams GR, Baldwin KM, Yasui N, Kishi K, Takeda S (2004) Skeletal muscle gene expression in space-flown rats. FASEB J 18(3):522–524
Nwoye L, Mommaerts WF, Simpson DR, Seraydarian K, Marusich M (1982) Evidence for a direct action of thyroid hormone in specifying muscle properties. Am J Physiol 242(3):R401–R408
Ogawa T, Furochi H, Mameoka M, Hirasaka K, Onishi Y, Suzue N, Oarada M, Akamatsu M, Akima H, Fukunaga T, Kishi K, Yasui N, Ishidoh K, Fukuoka H, Nikawa T (2006) Ubiquitin ligase gene expression in healthy volunteers with 20-day bedrest. Muscle Nerve 34(4):463–469
Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL (1998) Disproportionate loss of thin filaments in human soleus muscle after 17-day bed rest. Muscle Nerve 21(10):1280–1289
Roy RR, Baldwin KM, Edgerton VR (1996) Response of the neuromuscular unit to spaceflight: what has been learned from the rat model. Exerc Sport Sci Rev 24:399–425
Thomason DB, Booth FW (1989) Influence of performance on gene expression in skeletal muscle: effects of forced inactivity. Adv Myochem 2:79–82
Udaka J, Ohmori S, Terui T, Ohtsuki I, Ishiwata S, Kurihara S, Fukuda N (2008) Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. J Gen Physiol 131(1):33–41
Yaffe D, Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270(5639):725–727
Zhao R, Du L, Huang Y, Wu Y, Gunst SJ (2008) Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem 283(52):36522–36531
Acknowledgments
The authors thank T. Nedachi for critical reading of the manuscript, and Y. Morioka and A. Kondo for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujita, H., Hirano, M., Shimizu, K. et al. Rapid decrease in active tension generated by C2C12 myotubes after termination of artificial exercise. J Muscle Res Cell Motil 31, 279–288 (2010). https://doi.org/10.1007/s10974-010-9230-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-010-9230-9