Abstract
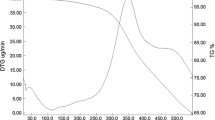
The curcumin is the major constituent of turmeric plant (Curcuma longa), which presents several biological activities such as antioxidant, bactericidal, anti-inflammatory and antitumor. An interesting strategy to improve its properties is to synthetize curcumin derivatives. Thus, the synthesis of a new curcumin derivative (DCUR) with 4-carboxybenzaldehyde (4-CBA) becomes interesting, which occurs by a Knoevenagel reaction, a common methodology used to synthesize curcumin analogues. The synthesis was carried out by the mechanochemical method, which is an efficient green method for the synthesis of organic compounds. The most efficient grinding time for mechanochemical synthesis was determined using PXRD and DSC techniques. 1H NMR, DEPTQ and FTIR analyses confirmed the structure of DCUR. This new methodology is more efficient (at least 5×), and cleaner than usual methods described in the literature to obtain a new compound by Knoevenagel reaction. The TG–DTA, DSC and DSC-microscopy techniques were used to confirm the formation of the compound and to study its thermal behavior. Moreover, the thermoanalytical of 4-CBA showed that this compound presents a reversible solid–solid phase transition.







Similar content being viewed by others
References
Gupta SC, Patchva S, Aggarwa BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013. https://doi.org/10.1208/s12248-012-9432-8.
Qiu X, et al. Synthesis and identification of new 4-arylidene curcumin analogs as potential anticancer agents targeting nuclear factor-κB signaling pathway. J Med Chem. 2014. https://doi.org/10.1021/jm1004545.
Aggarwal BB, et al. Curcumin—biological and medicinal properties. Tumeric Genus Curcuma. 2006;70:297–368.
Yousefi A, et al. Novel curcumin-based pyrano[2,3-d]pyrimidine anti-oxidant inhibitors for α-amylase and α-glucosidase: implications for their pleiotropic effects against diabetes complications. Int J Biol Macromol. 2015;78:46–55.
Ajavakom V, et al. Curcuminoids in multi-component synthesis. J Heterocycl Chem. 2018;55:13–20.
Kant V, et al. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int Immunopharmacol. 2014;20:322–30.
Submhan MA, et al. Synthesis and characterization of metal complexes containing curcumin (C21 H20 O6) and study of their anti-microbial activities and DNA binding properties. J Sci Res. 2015;6:97–109.
Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19:20091–112.
Santiago VH, et al. Curcumina. O pó dourado do açafrão-da-terra: Instrospecções sobre química e atividade biológicas. Quim Nova. 2010;33:538–52.
Hussain Z, et al. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: a review of new trends and future perspectives. Mater Sci Eng C. 2017;77:1316–26.
Bansal SS, et al. Advanced drug-delivery systems of curcumin for cancer chemoprevention. Cancer Prev Res. 2012;4:1158–71.
Ali I, et al. Curcumin-I Knoevenagel’s condensates and their Schiff’s bases as anticancer agents: synthesis, pharmacological and simulation studies. Bioorg Med Chem. 2013;21:3808–20.
Dohutia C, et al. Molecular docking. Synthesis and in vitro antimalarial evaluation of certain novel curcumin analogues. Braz J Pharm. 2007;3:1–14.
Knoevenagel E. Condensationen zwischen Malonester und Aldehyden unter dem Einfluss von Ammoniak und organischen Aminen. Ber Dtsch Chem Ges. 1895;27:2585–95.
Zambre AP, et al. Cooper Conjugates of Knoevenagel condesates of curcumin and theis Schiff Base derivatives: synthesis, spectroscopy, magnetism, ESR, and electrochemistry. Synth React Inorg, Metal Org Nano Met Chem. 2007;37:37–9.
Jha NS, et al. Targetin human telomeric G-quadruplex DNA with curcumin and its synthesized analogues under molecular crowding condition. RSC Adv. 2016;6:7474–87.
Khare R, et al. The importance and applications of knoevenagel reaction (brief review). Orient J Chem. 2018. https://doi.org/10.13005/ojc/350154.
Padhye S, et al. New difluoro knoevenagel condensates of curcumin, their schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm Res. 2009;26:1874–80.
Liu W, et al. Difluoroborate-based conjugated organic polymer: a high-performance heterogeneous photocatalyst for oxidative coupling reactions. J Mater Sci. 2019;54:1205–12212.
Haferkamp S, Kraus W, Emmerling F. Studies on the mechanochemical Knoevenagel condensation of fluorinated benzaldehyde derivates. J Mater Sci. 2018;53:13713–8.
Bowmaker GA, et al. Solvent-assisted mechanochemical synthesis of metal complexes. Dalt Trans. 2008;22:2926.
Hutchings BP, et al. Feedback Kinetics in Mechanochemistry: the Importance of Cohesive States. Angew Chem Int Ed. 2017. https://doi.org/10.1002/anie.201706723.
Achar TK, Bose A, Mal P. Mechanochemical synthesis of small organic molecules. Beilstein J Org Chem. 2017;13:1907–31.
Miao YR, Suslick KS. Mechanochemical reactions of metal-organic frameworks. Adv Inorg Chem. 2018;71:403–34.
Margetić D, Štrukil V. Mechanochemical organic synthesis. 1st ed. Amsterdam: Elsevier; 2016.
Leonardi M, Villacampa M, Menéndez JC. Multicomponent mechanochemical synthesis. Chem. Sci. 2018;9:2042–64.
Friscic T, Mottillo C, Titi HM. Mechanochemical for synthesis. Angew Chem Int Ed. 2019. https://doi.org/10.1002/anie.201906755.
Do J, Friscic T. Mechanochemistry: a force of synthesis. ACS Cent Sci. 2016;3:13–9.
Xu C, et al. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem Commun. 2015;51:6698–713.
Anastas P, Eghbali N. Green chemistry: principles and pratice. Green Chem Chem Sci. 2018;39:2042–64.
Piras CC, Fern S, De Borggraeve WM. Ball milling: a green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019;1:937–47.
Almeida AC, et al. Cocrystals of ciprofloxacin with nicotinic and isonicotinic acids: mechanochemical synthesis, characterization, thermal and solubility study. Thermochim Acta. 2020;685:1–10.
Rišianová L, et al. Synthesis, structural characterization and biological activity of novel Knoevenagel condensates on DLD-1 human colon carcinoma. Bioorg Med Chem Lett. 2017;27:2345–9.
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR. Introduction to spectroscopy. 5th ed. Stamford: Cengage; 2015.
Kolev TM, et al. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int J Quantum Chem. 2005;102:1069–79.
Nascimento M, et al. Physical and morphological properties of hydroxypropyl methylcellulose fi lms with curcumin polymorphs. Food Hydrocoll. 2019;97:105217.
Xavier TS, et al. Molecular and biomolecular spectroscopy vibrational spectral investigations and density functional theory study of 4-formylbenzoic acid. Spectrochim Acta Part A. 2013;114:502–8.
Guerra RG, et al. Thermal behaviour of curcumin. Braz J Therm Anal. 2012;1:19–23.
Chen Z, et al. Thermal degradation kinetics study of curcumin with nonlinear methods. Food Chem. 2014;155:81–6.
Haisa BYM, et al. Topochemical studies. VIII. The crystal and molecular structures of two polymorphs of 4-formylbenzoic acid. Acta Cryst. 1975;32:857–60.
Acknowledgements
The authors thank FAPESP (Proc. Nos. 2018/24378-6, 2018/14506-7 and 2018/12463-9) e CNPq (Proc. Nos. 421469/2016-1 and 302769/2018-8) and to CEPID- CDMF laboratory (Proc. FAPESP No. 2013/07296-2) for the X-ray Powder Diffraction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Moura, A., Gaglieri, C., da Silva-Filho, L.C. et al. Mechanochemical synthesis, characterization and thermoanalytical study of a new curcumin derivative. J Therm Anal Calorim 146, 587–594 (2021). https://doi.org/10.1007/s10973-020-10000-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10000-w