Abstract
The effect of different processing stages of dark and milk chocolate samples on their thermal properties was investigated. The ingredients of chocolates were investigated too. Thermal behavior was evaluated by means of thermogravimetry (TG) and first-derivative thermogravimetry (DTG). The measurements were made at a temperature range of 50–700 °C with a heating rate of 10 °C min−1. TG and DTG are thermal techniques, which have been taken advantage of research cocoa liquor, sugar, and cocoa butter in chocolates during the different processing stages. The obtained results evidenced possibility of indicating differences between milk fat and cocoa butter contribution and content of cocoa liquor and sugar which differ for dark and milk chocolates.
Similar content being viewed by others
Introduction
In many processed foods, the product structure depends not only on the ingredient formulation but also on the processing history of the material. This is particularly true for chocolate molding during which the fat component (cocoa butter for dark chocolate and cocoa butter and milk fat for milk chocolate) solidifies [1]. Confectionery products consist of proteins, sugars, and fats. Since the fat accounts for almost one-third for the weight of whole chocolate, its nature significantly determines the properties of the final product. Hence, fat phase is considered to be responsible, inter alia, for heat stability, mouth feel, flavor release, and general consumer satisfaction [2].
There are different types of chocolates (dark, milk, and white), according to their composition in terms of cocoa solids, milk fat, and cocoa butter, and hence the final products have different compositions [3]. Dark chocolates are suspensions of sucrose and cocoa particles, coated in phospholipids in a continuous fat phase, generally cocoa butter, with total solid content of 65–75%. The outcome is production of a smooth suspension of particulate solids in cocoa butter [4]. Dark chocolate is a complex food product in which sugar crystals and cocoa particles are surrounded by a continuous phase of crystalline and liquid cocoa butter. Due to the hydrophilic nature of the sugar crystals, a small portion of emulsifier, e.g., soy lecithin or polyglycerol polyricinoleate, is often added to improve compatibility with the hydrophobic cocoa butter [3, 5, 6]. During chocolate manufacture, tempering—a technique of controlled pre-crystallization, is used to induce a more thermodynamically stable polymorphic form of cocoa butter to effect good product snap, contraction, gloss, and shelf-life characteristics [7]. Important physical and functional characteristics (i.e., texture, snap and gloss) of chocolate products are dictated by the crystal network formed by its constituent lipid during crystallization [8]. Quality and stability of final products are affected by a number of factors, such as particle size, particle size distribution, and interaction between them, and are strictly related to the raw materials but also to the manufacture process [9,10,11,12,13]. The different process steps (mixing, pre-refining, refining, conching, and tempering) of chocolate manufacture and the different adopted process parameters can affect chocolate properties that, in turn, determine the behavior and the characteristics of the final product. An effective control of the technological parameters is required in order to achieve a constant and desirable quality of the final product [14, 15]. During chocolate manufacturing, mixtures of sugar, cocoa, and fat are heated, cooled, pressurized, and refined [6, 13]. Milk chocolate is a complex rheological system having solid particles (cocoa, milk powder, and sugar) dispersed in cocoa butter, which represents the fat phase [16]. The processing of milk chocolate involves during each single step (mixing, pre-refining, refining, conching, and tempering), modifications in its final quality and attributes, influencing in a strong way the microstructure of the product (aggregation, de-aggregation, reduction in particle size, immobilization of cocoa butter, etc.) [17,18,19,20]. In particular, milk powder with its own physical characteristics and inner porosity may have a significant impact on the chocolate-processing conditions and on the physical and organoleptic properties of the final product [21]. A deep knowledge of the influence of single processing stage on structural chocolate properties could be very useful and is a starting point if it is necessary to improve or modify final product characteristics [13].
Fast and sensitive methodologies of food analysis, especially for industrial purposes, are useful tools to determine the quality of final commercial products. Moreover, the possibility to use always easier tools to analyze the authenticity of the certificated food composition is a daily challenge. Thermal analysis is recognized as instrumental method of food analysis able to give unique information regarding the nature of the sample or the modifications induced by industrial processing. Books and reviews report the applications of thermoanalytical techniques to the food science [22,23,24,25,26,27,28]. Thermal analysis has been extensively applied to characterize the thermal, structural, and rheological properties of the chocolates [3, 8,9,10].
For this purpose, in the present work, the influence of each process phase on thermal properties of dark and milk chocolates and their ingredients were evaluated during the overall manufacturing process.
The purpose of the study was to assess the thermal properties of milk and dark chocolates, and ingredients used in chocolates production by thermogravimetry (TG) at nitrogen and oxygen flow.
Materials and method
Materials
Dark and milk chocolate samples were produced in Polish confectionery factory. Cocoa butter, milk fat, sugar, and cocoa liquor have been provided by the chocolate manufacturer. The chocolates were produced in an industrial plant provided of mixer, pre-refiner, refiner, conching, and tempering machine. Dark and milk chocolate production was made up by different steps: mixing, pre-refining, refining, conching, tempering and cooling, demoulding, and packing. The formulation employed for the recipe was cocoa solids (64%) (cocoa liquor and fat-reduced cocoa powder), sugar (35%), cocoa butter (32%), soy lecithin (0.3%), sodium carbonate (0.02%), fiber (13%), and vanilla extract (0.03%). The ingredients used in the milk chocolate formulation were: sugar (58%), cocoa butter (30%), whole milk powder (22%), whey milk powder (15%), sodium carbonate (0.02%), fiber (2.5%), and cocoa solids (30%) (cocoa liquor and fat-reduced cocoa powder). Experimental samples were taken after following production phases: refining, conching (before tempering), and packing. Samples were stored in plastic bucket (0.5 kg capacity) at room temperature until the analytical determinations.
Method
The analyses were performed using a Discovery TGA (TA Instruments) thermogravimetric analyzer. Measurements were made under nitrogen and oxygen at a flow rate of 25 mL min−1. Samples were placed in platinum containers. The mass of the sample loaded to the thermobalance was varied in the range of 6–9 mg. The range of operation was from 50 to 1000 °C, and measurements were made at a temperature range of 50–700 °C with heating rates of 2, 5, 10, and 15 °C min−1. TG curves were obtained for temperature dependence on mass loss, and first-derivative data (DTG) were calculated [3, 29]. All analyses were completed in triplicate.
The method has been validated on real samples at three selected temperatures in triplicate. The experimental extended uncertainty reached maximum 2%.
Results and discussion
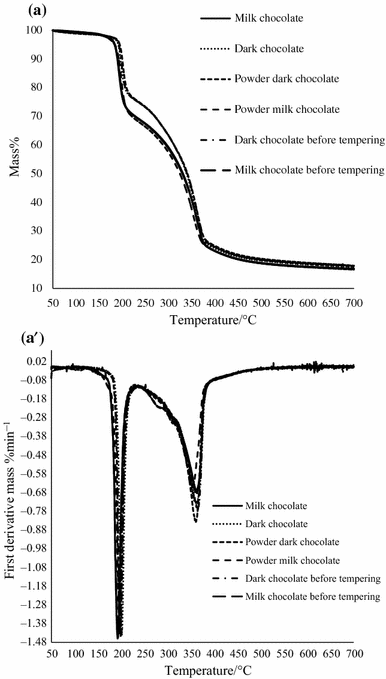
Thermogravimetric curves were determined and the first derivative for all samples was calculated. In Figs. 1, 2 and 3, TG and DTG curves of dark and milk chocolates of different processing stages in nitrogen with different heating rates 2, 5, and 15 °C min−1 are presented. Heating rate of 10 °C min−1 was representative for analysis according to Materazzi et al. [3]. Materazzi et al. [3] proved that heating rate of 10 min−1 is the best resolution for such experiments. The results of cocoa butter, milk fat, cocoa liquor, and sugar presented in Fig. 4 were obtained from TG and DTG analyses in nitrogen and oxygen. The TG curve of cocoa butter in nitrogen is characterized by only one step of decomposition (Fig. 4a). The peak in DTG curve at the temperature of 414 °C was observed. On the TG curve for cocoa butter, one transition in the range of 310–440 °C was detected. It was due to thermal degradation of cocoa butter [3]. Cocoa butter TG curve in oxygen is characterized by different course. The TG curve can be divided into four stages. First stage ranged from 50 to 290 °C, second—from 290 to 335 °C, third—from 335 to 420 °C, and fourth—from 420 to 700 °C. Different polymorphic forms, which are typical for cocoa butter, have very different physical properties but, upon melting, give identical liquids. In 1966, a complete study of the polymorphic states in cocoa butter was conducted, and the existence of the following polymorphic forms in order of increasing stability: I (sub-α or γ), II (α), III, IV (β′), V (β), and VI was determined. Form V (β) makes that cocoa butter remains stable for a very long period of time at the proper storage temperature [30]. On the DTG curve, four peaks were observed (Fig. 4a′). The first peak in DTG curve was detected at the temperature of 286 °C. The TG and DTG curves of milk fat in nitrogen and oxygen are presented in Fig. 4b, b′. The maximum temperature of first-derivative peak of milk fat was 384 °C in nitrogen. The course of milk fat curve was very similar to TG curve of cocoa butter. The profiles of the TG and DTG curves in oxygen showed five stages of decomposition of milk fat, with maximum temperature at 95, 296, 340, 414, and 511 °C. Sbihi et al. [31] obtained similar results by studying goat milk fat under dry air atmosphere. Milk fat due to its structure is polymorphic fat [32]. The fat in the liquid state reached by rapid cooling proceeds in the form of α, slow heating, and then solidifying causes the passage in the form of β′. Form β′ is more stable than form α. In an analogous manner, form β from the mold β′ is created. Form β is the most stable form of fat. There is also a fourth form γ (sub-α); it is characterized by a lower melting point than the form α and β′2 and directly converts in the form of β′ [31]. Milk fat may crystallize taking on three different polymorphic forms: γ, α, and β′. The most stable form of the milk fat is a form of β′ and the least—form γ [32,33,34]. Szabo et al. [35] reported that the temperature range corresponding to the first stage could be attributed to the thermal decomposition of the unsaturated fatty acids. The next stage phases represent the decompositions of trans isomers of fatty acids and saturated fatty acids. The thermogravimetric data TG and first-derivative data DTG of the cocoa liquor under a nitrogen and oxygen are shown in Fig. 4c, c′. The TG curves showed that cocoa liquor was thermally unstable in nitrogen and in oxygen. The course of the TG and DTG curves in nitrogen presented three stages of decomposition for cocoa liquor. First stage ranged from 50 to 325 °C, second—from 325 to 400 °C, and third—from 400 to 700 °C. The maximum temperatures at 289 and 390 °C were observed. Four stages of decomposition on TG and DTG curves of cocoa liquor in oxygen at maximum temperature: 253, 320, 447, and 491 °C were shown. Materazzi et al. [3] studied cocoa liquor in air flow and obtained similar results. The cocoa liquor is a mixture of cocoa butter, cocoa powder, cocoa solid, antioxidant flavor, and mineral compounds. The temperature of decomposition was corresponding with thermal degradation of ingredients of cocoa liquor. The thermogravimetric behavior of crystal sugar is shown in Fig. 4d, d′. In the TG curve of sugar in nitrogen, between 220 and 700 °C, continuous mass loss was observed. In TG curve in oxygen, three stages were observed that indicate changes in the rate of mass loss during thermogravimetric drying. This result can be attributed to water elimination from several sources. The type and degree of binding are reflected by the temperature of mass loss [36]. According to Iqbal et al. [37], the major weight loss (18–36%) occurred in the range of 225–325 °C, which was due to major degradation of the polysaccharide structure. Iqbal et al. [37] studied polysaccharides with the use of thermal analysis (TG, DTG, and DSC) in nitrogen. The DTG curves of sugar in nitrogen and in oxygen were characterized by two peaks at similar temperature for both gases. The first peaks with maximum temperatures of 225 and 228 °C in nitrogen and oxygen, respectively, were followed by the second—at 288 and 271 °C (Fig. 4d′). According to Roos et al. [38], the first event was attributed to sucrose melting and the second event was attributed to the elimination of volatile products (water, carbon monoxide, carbon dioxide, and hydrogen) resulting from the degradation of sucrose [36, 39]. Due to the complex behavior of sugars when melted, thermal decomposition reactions can occur before or close to the melting point, and in the literature a range of values for sucrose melting varies between 185 and 190 °C, yet it never reaches 225 °C [36, 38, 40]. The sugar’s origin may be responsible for the wide variation of melting points, since there are different sources (e.g., cane and beet sugar) and manufacturing methods. These findings of variation in sucrose melting peaks have mainly been related to impurities or polymorphism [36, 38, 41]. The DTG curve in oxygen showed peak at maximum temperature of 518 °C, which indicates the sugar sample decomposition.
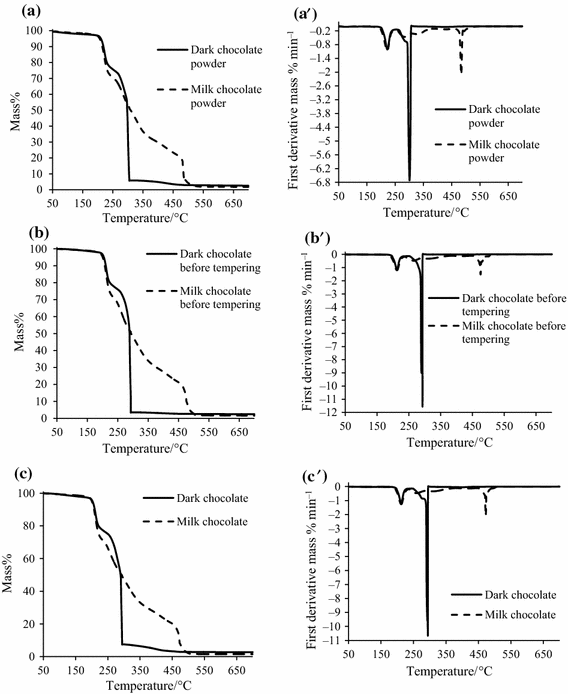
The thermogravimetric data (TG) and first-derivative data (DTG) of the three stages dark and milk chocolates production under heating in nitrogen atmosphere are presented in Fig. 5. Milk/dark chocolate powders are the products after refining process, milk/dark chocolate before tempering are the products after conching process, and dark/milk chocolates are the final chocolate bars. The TG curves that characterize samples of dark and milk chocolates at different stages of production can be divided into three steps. First stage ranged from 50 to 210 °C, second—from 210 to 350 °C, and third—from 350 to 700 °C. Materazzi et al. [3] stated that, by the analysis of the starting materials (sugar, cocoa liquor, and cocoa butter), it is very easy to assign each TG step in the dark chocolate curve: The first one is related to the sugar contribution, the second step is the release of the cocoa liquor, and the final is related to the mass loss of cocoa butter (Figs. 4 and 5). In the second step in TG curves of all production stages, the differences between milk and dark chocolates were observed (Fig. 5a–c). This phenomena indicated that amount of cocoa liquor in dark chocolate (powder, before tempering and chocolate bar) was higher. The dark chocolate powder TG curve showed higher rate of mass loss at third step than others TG curves. There were no differences in TG curves courses for different stages of dark/milk chocolates production, except of the TG course of dark chocolate powder. The first derivatives designated for fats and chocolates allowed identification of chocolate ingredients (Fig. 5a′–c′). In the case of the first peaks on DTG curves, for all samples, a temperature ranged from about 211–214 °C. The first peak proved sugar melting. The first peaks were more distinct for samples of milk chocolate at every production stage than for dark chocolate samples. Milk chocolate was characterized by higher amount of sugar in composition. In dark chocolate (for all stages of production) at a temperature ranged from 241 to 348 °C, the first derivative exhibits the presence of cocoa liquor, so no distinct peaks are observed for milk chocolate in this temperature region (Fig. 5a′–c′). The third peaks on DTG curves for all samples can be observed at temperature ranged from about 387–391 °C. The intensity of those peaks was more distinct for dark chocolates at all of production stages, because the content of cocoa butter was much higher than in milk chocolate. Peaks characterizing fat in milk chocolate do not have such a clear course.
The nitrogen-purging flow gives a clear qualitative profile, but the quantitative interpretation of the analysis is not allowed since inert flows depress the complete decomposition, without a final constant mass value [3]. By changing the atmosphere to oxygen, a final decomposition is obtained. There were differences in the TG curves and first derivatives for the chocolate powders, chocolate before tempering, and the finished dark and milk chocolate, in oxygen. The results of the samples analysis in oxygen for the three stages of dark and milk chocolates production are presented in Fig. 6. The shapes of TG curves showed three stages for dark and milk chocolates of different processing stages. The first transition was observed in the range of 50–230 °C (dark and milk chocolates), the second—230–300 °C (dark chocolate) and 230–500 °C (milk chocolate), and the third one—300–700 °C (dark chocolate), 500–700 °C (milk chocolate). The mass loss occurred with high rate for dark chocolate products than for milk chocolate products (Fig. 6a–c). The first peaks maximum temperature range corresponds to the thermal decomposition of sugar on all of DTG curves (Fig. 6a′–c′). The second transition was observed only for dark chocolates products. The peaks course indicated that the cocoa butter was oxidized. The DTG curves in oxygen for all dark chocolate products showed peak at maximum temperature range from 291 to 294 °C. Peaks characterizing fat in milk chocolate were characterized by less intensity. The third melting transition was observed on DTG curves of milk chocolate products. The last peaks represent the decomposition of sugar, like in Fig. 4d′. The maximum temperatures were observed at 493 (milk chocolate powder), 500 (milk chocolate before tempering), and 473 °C (milk chocolate). According to Glicerina et al. [20], the modifications in the microstructure of milk chocolate during the different processing steps involve deep changes in the rheological and colorimetric parameters of product. Rheological, textural, and thermal properties of dark chocolate are strictly related to the different steps of the manufacturing process [42].
Conclusions
TG and DTG are thermal techniques, which have been taken advantage of research cocoa liquor, sugar, and cocoa butter in chocolates during the different processing stages. The obtained results evidenced possibility of indicating differences between milk fat and cocoa butter and content of cocoa liquor and sugar (differences between dark and milk chocolates). TG and DTG investigations proved to be useful for research showing an adulteration of chocolates, especially fats’ adulteration.
References
Stapley A, Tewkesbury H, Fryer P. The effects of the shear and temperature history on the crystallization of chocolate. J Am Oil Chem Soc. 1999;76(6):677–85.
Kerti K. Investigating isothermal DSC method to distinguish between cocoa butter and cocoa butter alternatives. J Therm Anal Calorim. 2001;63:205–19.
Materazzi S, De Angelis Curtis S, Vecchio Ciprioti S, Risoluti R, Finamore J. Thermogravimetric characterization of dark chocolate. J Therm Anal Calorim. 2014;116:93–8.
Afoakwa E, Paterson A, Fowler M, Vieira J. Characterization of melting properties in dark chocolates from varying particle size distribution and composition using differential scanning calorimetry. Food Res Int. 2008;41:751–7.
Garti N, Wildak NR, editors. Cocoa butter and related compounds. Urbana: AOCS publishing; 2012.
Beckett ST. The science of chocolate. Cambridge: The Royal Society of Chemistry; 2000.
Afoakwa E, Paterson A, Fowler M, Vieira J. Influence of tempering and fat crystallization behaviours on microstructural and melting properties in dark chocolate systems. Food Res Int. 2009;42:200–9.
Afoakwa E, Paterson A, Fowler M. Factors influencing rheological and textural qualities in chocolate—a review. Trends Food Sci Technol. 2007;18:290–8.
Servais C, Jones R, Roberts I. The influence of particle size distribution on the processing of food. J Food Eng. 2002;51:201–8.
Granger C, Legerb A, Bareyb P, Langendorffb V, Cansell M. Influence of formulation on the structural networks in ice cream. Int Dairy J. 2005;15:255–62.
Sato A, Cunha R. Effect of particle size on rheological properties of jaboticaba pulp. J Food Eng. 2009;91:566–70.
Baldino N, Gabriele D, Migliori M. The influence of formulation and cooling rate on the rheological properties of chocolate. Eur Food Res Technol. 2010;231:821–8.
Glicerina V, Balestra F, Dalla Rosa M, Bergenhstål B, Tornberg E, Romani S. The influence of different processing stages on particle size, microstructure and appearance of dark chocolate. J Food Sci. 2014;79:1359–65.
Muller-Fischer N, Windhab E. Influence of process parameters on microstructure of food foam whipped in a rotor–stator device within a wide static pressure range. Colloids Surf A. 2005;263:353–62.
Baixauli R, Sanz T, Salvadora A, Fiszmana S. Influence of the dosing process on the rheological and microstructural properties of a bakery product. Food Hydrocolloid. 2007;21:230–6.
Pajin B, Dokic L, Zaric D, Šoronja-Simovic D, Loncarevic I, Nikolic I. Crystallization and rheological properties of soya milk chocolate produced in a ball mill. J Food Eng. 2013;114:70–4.
Aguilera J, Stanley D. Examining food microstructure. In: deMan JM, editor. Microstructural principles of food processing and engineering. 2nd ed. Gaithersburg: Aspen Publishers Inc.; 1999. p. 1–43.
Aguilera J, Stanley D, Baker K. New dimensions in microstructure of food products. Trends Food Sci Technol. 2000;11:3–9.
Afoakwa E, Paterson A, Fowler M, Vieira J. Microstructure and mechanical properties related to particle size distribution and composition in dark chocolate. Int J Food Sci Technol. 2009;44:111–9.
Glicerina V, Balestra F, Dalla Rosa M, Romani S. Effect of manufacturing process on the microstructural and rheological properties of milk chocolate. J Food Eng. 2015;145:45–50.
Liang B, Hartel R. Effects of milk powders in milk chocolate. J Dairy Sci. 2004;87:20–31.
Kemp R, editor. Handbook of thermal analysis and calorimetry: from macromolecules to man—chapter 16: thermal analysis in foods and food processes. Amsterdam: Elsevier; 1999.
Gabbot P, editor. Principles and applications of thermal analysis. Oxford: Wiley; 2007.
Materazzi S, Vecchio S. Recent applications of evolved gas analysis by infrared spectroscopy (IR-EGA). Appl Spectrosc Rev. 2013;48(8):654–89.
Materazzi S, Vecchio S. Evolved gas analysis by mass spectrometry. Appl Spectrosc Rev. 2011;46(4):261–340.
Materazzi S, Vecchio S. Evolved gas analysis by infrared spectroscopy. Appl Spectrosc Rev. 2010;45:241–73.
Materazzi S, Gullifa G, Fabiano M, Frati P, Santurro A, Scopetti M, Fineschi V, Risoluti R. New frontiers in thermal analysis. A TG/Chemometrics approach for postmortem interval estimation in vitreous humor. J Therm Anal Calorim. 2017;130:549–57.
Mohamad SNH, Muhamad II, Khairuddin N, Mohd Jusoh YM. Stability study of α-toc/β-CD powders obtained by microwave heating and encapsulation process. J Therm Anal Calorim. 2017;130:1473–80.
Albis A, Ortiz E, Suarèz A, Piňeres I. TG/MS study of the thermal devolatization of Copoazú peels (Theobroma grandiflorum). J Therm Anal Calorim. 2014;115:275–83.
Wille R, Lutton E. Polymorphism of cocoa butter. J Am Oil Chem Soc. 1966;43:491–6.
Sbihi H, Nehdi I, Tan C, Al-Resayes S. Characteristics and fatty acid composition of milk fat from Saudi Aradi goat. Grasas Aceites. 2015;66(4):1–8.
Ten Grotenhuis E, Van Aken G, Van Malssen K, Schenk H. Polymorphism of milk fat studied by differential scanning calorimetry and real-time X-ray powder diffraction. J Am Oil Chem Soc. 1999;76(9):1031–9.
Wright A, Hartel R, Narine S, Marangoni A. The effect of minor components on milk fat crystallization. J Am Oil Chem Soc. 2000;77(5):463–75.
Lopez C, Riaublanc A, Lesieur P, Bourgaux C, Keller G, Ollivon M. Definition of a model fat for crystallization-in-emulsion studies. J Am Oil Chem Soc. 2001;78(12):1233–44.
Szabo M, Chambre D, Iditoiu C. TG/DTG/DTA for the oxidation behaviour characterization of vegetable and animal fats. J Therm Anal Calorim. 2012;110:281–5.
Ducat G, Felsner M, da Costa Neto P, Quináia S. Development and in house validation of a new thermogravimetric method for water content analysis in soft brown sugar. Food Chem. 2015;177:158–64.
Iqbal M, Massey S, Akbar J, Ashraf C, Masih R. Thermal analysis of some natural polysaccharide materials by isoconversional method. Food Chem. 2013;140:178–82.
Ross Y, Karel M, Labuza T, Levine H, Mathlouthi M, Reid D, Shalaev E, Slade L. Melting and crystallization of sugars in high-solids systems. J Agric Food Chem. 2013;61:3167–78.
Kumaresan R, MoorthyBabu S. Crystal growth and characterization of sucrose single crystals. Mater Chem Phys. 1997;49:83–6.
Lee J, Thomas L, Jerrell J, Feng H, Cadwallader K, Schmidt S. Investigation of thermal decomposition as the kinetic process that causes the loss of crystalline structure in sucrose using a chemical analysis approach (part II). J Agric Food Chem. 2011;59:702–12.
Lee J, Thomas L, Schmidt S. Investigation of the heating rate dependency associated with the loss of crystalline structure in sucrose, glucose and fructose using a thermal analysis approach (part I). J Agric Food Chem. 2011;59:684–701.
Glicerina V, Balestra F, Dalla Rosa M, Romani S. Rheological, textural and calorimetric modifications of dark chocolate during process. J Food Eng. 2013;119:173–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ostrowska-Ligęza, E., Górska, A., Wirkowska-Wojdyła, M. et al. Thermogravimetric characterization of dark and milk chocolates at different processing stages. J Therm Anal Calorim 134, 623–631 (2018). https://doi.org/10.1007/s10973-018-7091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7091-4