Abstract
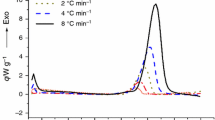
In this paper, the thermal stability and decomposition kinetics of 10 nitric esters including nitroglycerine (NG), pentaerythritol tetranitrate (PETN), trimethylolethane trinitrate (TMETN), dipentaerythritol hexanitrate (DiPEHN), trimethylolpropane trinitrate (TMPTN), xylitol pentanitrate (XPN), sorbitol hexanitrate (SHN), erythritol tetranitrate (ENT), mannitol hexanitrate (MHN) and nitroisobutylglycerol trinitrate (NiBGT) are investigated by non-isothermal TG and DSC. It has been shown that the mass loss processes of NG, TMETN and TMPTN are more dependent on the heating rate, and the simultaneous evaporation makes the initial temperatures for their mass loss lower than those of the other nitric esters. Based on the correlations among their thermal stability, activation energy, detonation velocity and heat of combustion, one could conclude that the oxygen coefficient (α) plays a positive role on the decomposition heat release efficiency (η d) when it is less than one, whereas when the α is greater than or equals to one, the fuel elements such as C and H contents would play a decisive role on η d. It has been further proved that the order of contribution rate of function groups on the tertiary carbon to the detonation velocity could be –CH3 < –NO2 < –C2H5 < –ONO2. In addition, the introduction of function groups to the tertiary carbon is in favor of increasing the thermal stability of nitric esters due to increase in symmetry and rigidity of their molecules. The proportion numbers (C s) of methylene group (–CH2–) to tertiary carbon or quaternary carbon will, to some extent, determine the thermal stability of the nitric esters.








Similar content being viewed by others
References
Manelis GB, Nazin GM, Rubtsov YI, Strunim VA. Chapter 9 in thermal decomposition and combustion of explosives and propellants. Taylor & Francis Group; 2003. ISBN 0-415-29984-5.
Yan Q-L, Zeman S, Elbeih A. Recent advances in thermal analysis and stability evaluation of insensitive plastic bonded explosives (PBXs). Thermochim Acta. 2012;537:1–12.
Oxley JC, Smith JL, Brady JE IV, Brown AC. Characterization and analysis of tetranitrate esters. Propellants Explos Pyrotech. 2012;37:24–39.
Shen S-M, Chang F-M, Hu J-C, Leu A-L. Thermal behaviour and compatibility of polyurethane PCL/BDNPA/F and PCL/TMETN blends. Thermochim Acta. 1991;181:277–88.
Chen P, Zhao F-Q, Li S-W. The study on the quenched surface characteristics of NC/TMETN propellant with potassium salt. Chin J Explos Propellants. 2002;25(3):47–50.
Brown GW, Sandstrom MM, Giambra AM, Archuleta JG, Monroe DC. Thermal analysis of pentaerythritol tetranitrate and development of a powder aging model. In: 37th annual conference of the North American Thermal Analysis Society, Lubbock, TX; 2009. Sept 20–23.
Elbeih A, Pachman J, Zeman S, Akštein Z. Replacement of PETN by bicyclo-HMX in Semtex 10. In: 8th international armament conference on scientific aspects of armament and safety technology, Pułtusk, Poland, vol. 2, no. 2; 2010. p. 7–16.
Wu S-D, Zhang Z-H, Li D-Y, Jin J-Z, Kong J, Tian Z, Wang W, Wang M-F. Nitroester drug’s effects and their antagonistic effects against morphine on human sphincter of Oddi motility. World J Gastroenterol. 2005;11(15):2319–23.
Zhao F-Q, Xu S-Y, Yi J-H, Gao H-X, Song H-C, Li S-W. Numerical simulation for combustion characteristics of insensitive propellant containing trimethylolethane trinitrate (TMETN). Chin J Explos Propellants. 2006;14(6):406–10.
Zhao F-Q, Chen P, Li S-W, Yin C-M, Luo Y. Interaction of potassium salt flame suppressors with TMETN and burning catalysts during decomposition. Energ Mater. 2001;9(3):102–3.
Lee J-S, Hsu C-K, Chang C-L. A study on the thermal decomposition behaviors of PETN, RDX, HNS and HMX. Thermochim Acta. 2002;392–393:173–6.
Li M-M, Wang G-X, Guo X-D, Wu Z-W, Song H-C. Theoretical studies on the structures, thermodynamic properties, detonation properties, and pyrolysis mechanisms of four trinitrate esters. J Mol Struct (Thoechem). 2009;900:90–5.
Roos BD, Brill TB. Thermal decomposition of energetic materials 82. Correlations of gaseous products with the composition of aliphatic nitrate esters. Combust Flame. 2002;128:181–90.
Li X, Tang Z, Zhang X, Yang X. The heats of formation in a series of nitroester energetic compounds: a theoretical study. J Hazard Mater. 2009;165(1–3):372–8.
Pachman J, Selesovsky J, Künzel M, Matyas R, Kubat K, Anastacio AC, Kuera J. Blast wave parameters of liquid esters of nitric acid: propane-1,2,3-triyl trinitrate, propane-1,2-diyl dinitrate, ethane-1,2-diyl dinitrate and methyl nitrate. Cent Eur J Energ Mater. 2017;14(2):375–90.
Zhang G-Y, Wei X-A, Du P. Effect of boron-containing hydrogen-storage-alloy (Mg(BHx)y) on the explosion energy of nitric ester explosive. Chin J Energ Mater. 2016;24(12):1205–8.
Keshavarz MH, Rahimi R, Akbarzadeh AR. Two novel correlations for assessment of crystal density of hazardous ionic molecular energetic materials using their molecular structures. Fluid Phase Equilib. 2015;402:1–8.
Manner VW, Tappan BC, Scott BL, Preston DN, Brown GW. Crystal structure, packing analysis, and structural-sensitivity correlations of erythritol tetranitrate. Cryst Growth Des. 2014;14(11):6154–60.
Yan Q-L, Künzel M, Zeman S, Svoboda R, Bartosková M. The effect of molecular structure on thermal stability, decomposition kinetics and reaction models of nitric esters. Thermochim Acta. 2013;566:137–48.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Manelis GB, Nazin GM, Rubtsov YI, Strunin VA. Thermal decomposition and combustion of explosives and propellants. New York: Taylor & Francis; 2003. p. 363.
Waring CE, Krastins G. The kinetics and mechanism of the thermal decomposition of nitroglycerin. J Phys Chem. 1970; 74(5):199.
Robertson R. The velocity of decomposition of nitroglycerin by heat. Part I. J Chem Soc. 1909;95:1241–8.
Svetov BS. Thermal decomposition of nitro esters. Dissertation, Doctor of chemical science, Moscow, Mendeleev Chemical Technical Institute. 1970; p. 277.
Phillips L. Thermal decomposition of organic nitrates. Nature. 1947;160:763.
Ellis WR, Smythe BM, Treharne ED, editors. 5th international symposium on combustion. 1955; p. 641.
Klimenko GK. Goreniye I vzryuv (combustion and explosion). In: 4th all-union symposium on combustion and explosion, Izdat, Nauka, Moscow, 1977; p. 587.
Andreev KK, Kaidymov BI. Thermal decomposition of nitrate esters. II. Thermal decomposition of pentaerythritol tetranitrate. In: Andreev K, Moscow KK, editors. Explosive theory; 1963. p. 241.
Robertson AIB. The thermal decomposition of pentaerythritol tetranitrate, nitroglycerin, ethylenediamine dinitrate and ammonium nitrate. J Soc Chem Ind. 1948;67:221.
Goldbinder. Laboratory practice in the theory of explosives (in Russ.), Rosmzidat, Moscow; 1963. p. 1–9.
Zeman S, Dimun M, Truchlikt S. The relationship between kinetic data of low-temperature thermal analysis and the heats of explosion of inorganic azides. Thermochim Acta. 1984;78:181.
Zeman S. New dependence of activation energies of nitroesters thermolysis and possibility of its application. Propellant Explos Pyrotech. 1992;17:17–9.
Zeman S, Elbeih A, Yan Q-L. Note on the use of the vacuum stability test in the study of initiation reactivity of attractive cyclic nitramines in Formex P1 matrix. J Therm Anal Calorim. 2013;111:1503–6.
Zeman S, Elbeih A, Yan Q-L. Notes on the use of the vacuum stability test in the study of initiation reactivity of attractive cyclic nitramines in the C4 matrix. J Therm Anal Calorim. 2013;112(3):1433–7.
Rogers RN. Thermochemistry of explosives. Thermochim Acta. 1975;11:131–9.
Zeman S. Sensitivities of high energy compounds. In: Klapoetke T, editor. High energy density materials, series: structure and bonding, vol. 125. New York: Springer; 2007. p. 195–271.
Zeman S. Modified Evans–Polanyi–Semenov relationship in the study of chemical micromechanism governing detonation initiation of individual energetic materials. Thermochim Acta. 2002;384:137–54.
Zeman S. New application of kinetic data of the low-temperature thermolysis of nitroparaffins. Thermochim Acta. 1995;261:195–207.
Mohammad HK, Bahman ES, Ali H. A new method for predicting the heats of combustion of polynitro arene, polynitro heteroarene, acyclic and cyclic nitramine, nitrate ester and nitroaliphatic compounds. J Hazard Mater. 2011;185:1086–106.
Wang G, Gong X, Du H, Liu Y, Xiao H. Theoretical prediction of properties of aliphatic polynitrates. J Phys Chem A. 2011;115:795–804.
Yi JH, Zhao FQ, Xu SY, Gao HX, Hu RZ. Thermal behavior, nonisothermal decomposition reaction kinetics of mixed ester double-base gun propellants. Chem Res Chin Univ. 2008;24(5):608–41.
Politzer P, Murray JS. Some perspectives on estimating detonation properties of C, H, N, O compounds. Cent Eur J Energ Mater. 2011;8(3):209–20.
Ou Y-X. Explos Mater Sci. Beijing University of Science and Technology Press: Beijing; 2006.
Acknowledgements
This work was supported by funding of “the Fundamental Research Funds for the Central Universities” with Project number of 3102017OQD003 and “Thousand Youth Talents Plan” with project code of 17GH030127.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dong, J., Yan, QL., Liu, PJ. et al. The correlations among detonation velocity, heat of combustion, thermal stability and decomposition kinetics of nitric esters. J Therm Anal Calorim 131, 1391–1403 (2018). https://doi.org/10.1007/s10973-017-6706-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6706-5