Abstract
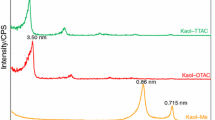
The structural property, thermal behavior, and morphology of octyltrimethylammonium chloride–kaolinite complexes prepared at different reaction temperatures were studied by X-ray diffraction, Fourier transform infrared spectroscopy, thermogravimetry–differential scanning calorimetry, and scanning electron microscope. The present study demonstrated that the arrangement model of octyltrimethylammonium cations (OTAC+) within the kaolinite interlayer space was independent of reaction temperature. The alkyl chains adopted a similar rigid paraffin-bilayer arrangement with different tilted angles. Although the intercalation led to an increased number of gauche conformers, the number of nonlinear conformers remained constant with increasing temperature. With increasing temperature, the number of trans conformers continuously augmented and resulted in decreased gauche/trans ratio. Therefore, the molecular environment remained solid like. Simultaneously, the surfactant packing density gradually increased, along with the decreasing water content in the organoclays. This effect improved thermal stability and hydrophobicity. The thermal decomposition processes of the kaolinite–OTAC+ complex can be divided into four steps. Furthermore, SEM images showed that the morphology of these complexes was strongly dependent on the given temperature. In general, increasing the temperature within the limited given temperature (≤70 °C) promoted the transformation from platy layers to nanoscrolls. Most of the transformed nanoscrolls were acquired in the products prepared at 70 °C, and further increasing in temperature decreased the nanoscrolls yield. Nevertheless, the packing density increased in the process, thereby demonstrating that the packing density not only promoted nanoscrolls transformation but also prevented the progress.








Similar content being viewed by others
References
Meier LP, Nueesch R, Madsen FT. Organic pillared clays. J Colloid Interface Sci. 2001;238(1):24–32.
Manias E, Hadziioannou G, ten Brinke G. Inhomogeneities in sheared ultrathin lubricating films. Langmuir. 1996;12(19):4587–93.
He H, Duchet J, Galy J, Gérard JF. Influence of cationic surfactant removal on the thermal stability of organoclays. J Colloid Interface Sci. 2006;295(1):202–8.
Choy JH, Kwak SY, Han YS, Kim BW. New organo-montmorillonite complexes with hydrophobic and hydrophilic functions. Mater Lett. 1997;33(3):143–7.
Wu J, Lerner MM. Structural, thermal, and electrical characterization of layered nanocomposites derived from sodium-montmorillonite and polyethers. Chem Mater. 1993;5(6):835–8.
Wang Z, Pinnavaia TJ. Nanolayer reinforcement of elastomeric polyurethane. Chem Mater. 1998;10(12):3769–71.
Cheng H, Liu Q, Zhang J, Yang J, Frost RL. Delamination of kaolinite-potassium acetate intercalates by ball-milling. J Colloid Interface Sci. 2010;348(2):355–9.
Cheng H, Zhang S, Liu Q, Li X, Frost RL. The molecular structure of kaolinite-potassium acetate intercalation complexes: a combined experimental and molecular dynamic simulation study. Appl Clay Sci. 2015;116:273–80.
Kwolek T, Hodorowicz M, Stadnicka K, Czapkiewicz J. Adsorption isotherms of homologous alkyldimethylbenzylammonium bromides on sodium montmorillonite. J Colloid Interface Sci. 2003;264(1):14–9.
Tang Y, Hu Y, Song L, Zong R, Gui Z, Chen Z, et al. Preparation and thermal stability of polypropylene/montmorillonite nanocomposites. Polym Degrad Stab. 2003;82(1):127–31.
Yilmaz N, Yapar S. Adsorption properties of tetradecyl- and hexadecyl trimethylammonium bentonites. Appl Clay Sci. 2004;27(3–4):223–8.
Lee JY, Lee HK. Characterization of organobentonite used for polymer nanocomposites. Mater Chem Phys. 2004;85(2–3):410–5.
Kozak M, Domka L. Adsorption of the quaternary ammonium salts on montmorillonite. J Phys Chem Solids. 2004;65(2–3):441–5.
Someya Y, Shibata M. Morphology, thermal, and mechanical properties of vinylester resin nanocomposites with various organo-modified montmorillonites. Polym Eng Sci. 2004;44(11):2041–6.
Favre H, Lagaly G. Organo-bentonites with quaternary alkylammonium ions. Clay Miner. 1991;26(1):19–32.
Lagaly G. Characterization of clays by organic compounds. Clay Miner. 1981;16:1–21.
Klapyta Z, Fujita T, Iyi N. Adsorption of dodecyl- and octadecyltrimethylammonium ions on a smectite and synthetic micas. Appl Clay Sci. 2001;19:5–10.
He H, Ma Y, Zhu J, Yuan P, Qing Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl Clay Sci. 2010;48(1–2):67–72.
Zhu J, He H, Guo J, Yang D, Xie X. Arrangement models of alkylammonium cations in the interlayer. Chin Sci Bull. 2003;4(48):368–72.
Bergaya F, Lagaly G. Handbook of clay science. 2nd ed. Amsterdam: Elsevier; 2013.
Vaia RA, Teukolsky RK, Giannelis EP. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem Mater. 1994;6:1017–22.
Heller L, Yariv S. Anilinium montmorillonites and the formation of ammonium/amine associations. Isr J Chem. 1970;8(3):391–7.
Kuroda Y, Ito K, Itabashi K, Kuroda K. One-step exfoliation of kaolinites and their transformation into nanoscrolls. Langmuir. 2011;27(5):2028–35.
Yuan P, Tan D, Annabi-Bergaya F, Yan W, Liu D, Liu Z. From platy kaolinite to aluminosilicate nanoroll via one-step delamination of kaolinite: effect of the temperature of intercalation. Appl Clay Sci. 2013;83–84:68–76.
Long H, Zheng Y, Guo Y, Li B. Preparation and characterization of kaolinite nanoscrolls. ChinJ InorgChem. 2012;28(6):1210–6.
Frost RL, Kristof J, Paroz GN, Kloprogge JT. Molecular structure of dimethyl sulfoxide intercalated kaolinites. J Phys Chem, B. 1998;102(43):8519–32.
Komori Y, Enoto H, Takenawa R, Hayashi S, Sugahara Y, Kuroda K. Modification of the interlayer surface of kaolinite with methoxy groups. Langmuir. 2000;18:5506–8.
Liu Q, Zhang S, Cheng H, Wang D, Li X, Hou X, et al. Thermal behavior of kaolinite-urea intercalation complex and molecular dynamics simulation for urea molecule orientation. J Therm Anal Calorim. 2014;117(1):189–96.
Tunney JJ, Detellier C. Interlamellar covalent grafting of organic units on kaolinite. Chem Mater. 1993;5(6):747–8.
Yui T, Yoshida H, Tachibana H, Tryk DA, Inoue H. Intercalation of polyfluorinated surfactants into clay minerals and the characterization of the hybrid compounds. Langmuir. 2002;18:891–6.
He H, Frost RL, Zhu J. Infrared study of HDTMA+ intercalated montmorillonite. Spectrochim Acta A. 2004;60(12):2853–9.
Yan L, Low PF, Roth CB. Swelling pressure of montmorillonite layers versus HOH bending frequency of the interlayer water. Clays Clay Miner. 1996;44(6):749–56.
Yan L, Roth CB, Low PF. Changes in the Si-O vibrations of smectite layers accompanying the sorption of interlayer water. Langmuir. 1996;12(18):4421–9.
Johnston C, Sposito G, Erickson C. Vibrational probe studies of water interactions with montmorillonite. Clays Clay Miner. 1992;40(6):722–30.
Madejova J, Janek M, Komadel P, Herbert H-J, Moog H. FTIR analyses of water in MX-80 bentonite compacted from high salinary salt solution systems. Appl Clay Sci. 2002;20(6):255–71.
Venkataraman N, Vasudevan S. Conformation of methylene chains in an intercalated surfactant bilayer. J Phys Chem, B. 2001;105(9):1805–12.
Hagemann H, Snyder R, Peacock A, Mandelkern L. Quantitative infrared methods for the measurement of crystallinity and its temperature dependence: polyethylene. Macromolecules. 1989;22(9):3600–6.
Kawai T, Umemura J, Takenaka T, Kodama M, Seki S. Fourier transform infrared study on the phase transitions of an octadecyltrimethylammonium chloride-water system. J Colloid Interface Sci. 1985;103(1):56–61.
Cameron DG, Umemura J, Wong PT, Mantsch HH. A Fourier transform infrared study of the coagel to micelle transitions of sodium laurate and sodium oleate. Colloids Surf. 1982;4(2):131–45.
Snyder R, Strauss H, Elliger C. Carbon-hydrogen stretching modes and the structure of n-alkyl chains. 1. Long, disordered chains. J Phys Chem. 1982;86(26):5145–50.
Ma Y, Zhu J, He H, Yuan P, Shen W, Liu D. Infrared investigation of organo-montmorillonites prepared from different surfactants. Spectrochim Acta A. 2010;76(2):122–9.
He H, Ding Z, Zhu J, Yuan P, Xi Y, Yang D, et al. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005;53(3):287–93.
Yariv S. The role of charcoal on DTA curves of organo-clay complexes: an overview. Appl Clay Sci. 2004;24(3–4):225–36.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (51034006), Beijing Natural Science Foundation (8164062), Beijing Nova Program (xx2015B081), and Beijing talent plan (2014000020124G164).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, D., Liu, Q., Cheng, H. et al. Effect of reaction temperature on intercalation of octyltrimethylammonium chloride into kaolinite. J Therm Anal Calorim 128, 1555–1564 (2017). https://doi.org/10.1007/s10973-016-6052-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6052-z