Abstract
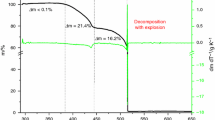
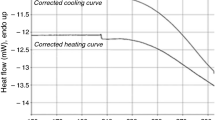
Thermal analysis (TG/DTG/QMS), performed for [Sr(OS(CH3)2)6](ClO4)2 in a flow of argon and in temperature range of 295–585 K, indicated that the compound is completely stable up to ca. 363 K, and next starts to decompose slowly, and in the temperature at ca. 492 K looses four (CH3)2SO molecules per one formula unit. During further heating [Sr(DMSO)2](ClO4)2 melts and simultaneously decomposes with explosion. Differential scanning calorimetry (DSC) measurements performed in the temperature range of 93–370 K for [Sr(DMSO)6](ClO4)2 revealed existence of the following phase transitions: glass ↔ crystal phase Cr5 at T g ≈ 164 K (235 K), phase Cr5 → phase Cr4 at \( T_{\text{c6}}^{\text{h}} \) ≈ 241 K, phase Cr4 → phase Cr3 at \( T_{\text{c5}}^{\text{h}} \) ≈ 255 K, phase Cr3 → phase Cr2 at \( T_{\text{c4}}^{\text{h}} \) ≈ 277 K, phase Cr2 ↔ phase Cr1 at \( T_{\text{c3}}^{\text{h}} \) ≈ 322 K and \( T_{\text{c3}}^{\text{c}} \) ≈ 314 K, phase Cr1 ↔ phase Rot2 at \( T_{\text{c2}}^{\text{h}} \) ≈ 327 K and \( T_{\text{c2}}^{\text{c}} \) ≈ 321 K and phase Rot2 ↔ phase Rot1 at \( T_{\text{c1}}^{\text{h}} \) ≈ 358 K and \( T_{\text{c1}}^{\text{c}} \) ≈ 347 K. Entropy changes values of the phase transitions at \( T_{\text{c1}}^{\text{h}} \) and \( T_{\text{c2}}^{\text{h}} \) (∆S ≈ 79 and 24 J mol−1 K−1, respectively) indicated that phases Rot1 and Rot2 are substantially orientationally disordered. The solid phases (Cr1–Cr5) are more or less ordered phases (∆S ≈ 7, 10, 4 and 3 J mol−1 K−1, respectively). Phase transitions in [Sr(DMSO)6](ClO4)2 were also examined by Fourier transform middle infrared spectroscopy (FT-MIR). The characteristic changes in the FT-MIR absorption spectra of the low- and high-temperature phases observed at the phase transition temperatures discovered by DSC allowed us to relate these phase transitions to the changes of the reorientational motions of DMSO ligands and/or to the crystal structure changes.






Similar content being viewed by others
References
Migdał-Mikuli A, Mikuli E, Szostak E, Serwońska J. Phase polymorphism of [Cd(DMSO)6](ClO4)2 studied by differential scanning calorimetry. Z Naturforsch A. 2003;58:341–5.
Migdał-Mikuli A, Szostak E. Phase polymorphism of [Co(DMSO)6](ClO4)2 studied by differential scanning calorimetry. Thermochim Acta. 2005;426:191–8.
Migdał-Mikuli A, Szostak E. Phase polymorphism of [Mn(DMSO)6](ClO4)2 studied by differential scanning calorimetry. Z Naturforsch A. 2005;60:289–95.
Migdał-Mikuli A, Szostak E. Phase polymorphism of [Zn(DMSO)6](ClO4)2 studied by differential scanning calorimetry. Thermochim Acta. 2006;444:195–200.
Migdał-Mikuli A, Szostak E. Phase polymorphism of [Ni(DMSO)6](ClO4)2 studied by differential scanning calorimetry. Z Naturforsch A. 2007;62a:67–74.
Szostak E, Migdał-Mikuli A. Phase polymorphism and thermal decomposition of hexadimethylsulphoxidemagnesium(II) chlorate(VII). J Therm Anal Calorim. 2010;101:601–6.
Migdał-Mikuli A, Szostak E, Nitek W. Hexakis(dimethylsulphoxide)manganese(II) bis(perchlorate). Acta Cryst E. 2006;62:m2581.
Szostak E, Migdał-Mikuli A, Kaczor A, Nitek W. Low-temperature phase transition in [Mn(OS(CH3)2)6](ClO4)2 studied by single crystal X-ray diffraction, infrared absorption and Raman scattering spectroscopies. Spectrochim Acta A. 2011;79:1179–86.
Persson I. The crystal structure of hexakis(dimethylsulphoxide)zinc(II) perchlorate and the structure of (dimethylsulphoxide)zinc(II) ion in dimethylsulfoxide solution. Acta Chem Scand. 1982;36a:7–13.
Calligaris M, Carugo O. Structure and bonding in metal sulfoxide complexes. Coord Chem Rev. 1996;153:83–154.
Chan EJ, Cox BG, Harrowfield JM, Ogden MI, Skelton BW, White AH. Cation solvation in the solid state-temperature-dependent crystal structures in some metal perchlorates solvated by dimethylsulfoxide. Inorg Chim Acta. 2004;357:2365–73.
Górska N, Inaba A, Szostak E, Mikuli E. Phase transitions and reorientational motions in [Al(OS(CH3)2)6](NO3)3 and its deuterated analog. Thermochim Acta. 2012;533:66–73.
Migdał-Mikuli A, Górska N, Szostak E. Phase transition and thermal decomposition of [Al(DMSO)6]Cl3. J Therm Anal Calorim. 2007;90:223–8.
Migdał-Mikuli A, Górska N. Thermal behaviour of [Mg(DMSO)6](NO3)2. J Therm Anal Calorim. 2007;90:833–9.
Raman/IR Atlas. Verlag Chemie GmbH Weinheim. Bergstr; 1974.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. 6 Part B ed. Hoboken: Wiley; 2009.
Szostak E, Drużbicki E, Mikuli E. Molecular structure and vibrational spectrum of [Mg((CH3)2SO)6](ClO4)2 studied by infrared and Raman spectroscopies and DFT computations. J Mol Struct. 2010;970:139–46.
Mikuli E, Migdał-Mikuli A, Majda D. Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2. J Therm Anal Cal. 2012. doi: 10.1007/s10973-012-2640-8.
Wunderlich B. A classification of molecules, phases and transitions as recognized by thermal analysis. Thermochim Acta. 1999;340–341:37–52.
Martınez Casado FJ, Ramos Riesco M, Redondo Yelamos MI, Sanchez Arenas A, Rodrıguez Cheda JA. The role of calorimetry in the structural study of mesophases and their glass states. J Therm Anal Calorim. 2012;108:399–413.
Berney CV, Weber JH. Complexes of sulfoxides. II. Metal-oxygen stretching vibrations in complexes of dimethyl sulfoxide and dimethyl sulfoxide-d 6 and point group of the cation. Inorg Chem. 1968;7:283–7.
Acknowledgments
The research was carried out with the equipment purchased, thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Migdał-Mikuli, A., Szostak, E. & Bernard, P. Thermal analysis, phase transitions and molecular reorientations in [Sr(OS(CH3)2)6](ClO4)2 . J Therm Anal Calorim 115, 443–449 (2014). https://doi.org/10.1007/s10973-013-3209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3209-x