Abstract
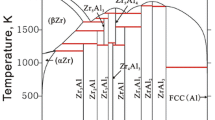
The Al-rich phase equilibria in the Al–Zr binary system were investigated experimentally. The phase diagram for compositions up to 40 at.% Zr was determined experimentally by differential thermal analysis and metallography. Three stable intermetallic compounds exist in this region of the diagram: Al3Zr2, Al2Zr, and Al3Zr. The peritectic melting of Al3Zr2 and the congruent melting of Al2Zr were confirmed. Al3Zr, the most Al-rich intermetallic compound, melts peritectically, which contradicts information available in the literature. In addition, the reaction between Al3Zr and the (Al) solid solution seems to be of eutectic nature, in contradiction with previous results found in the literature. Based on these new experimental evidence, a revised phase diagram is drawn.







Similar content being viewed by others
References
Fink WL, Willey LA. Equilibrium relation in Al–Zr alloys. Met Technol. 1939;1:69–80.
McPherson DJ, Hansen M. The system Zr–Al. Trans ASM. 1954;46:354–74.
Chiotti P, Woerner PF. Metal hydride reactions: I. Reaction of hydrogen with solutes in liquid metal solvents. J Less Common Met. 1964;7:111–9.
Glazov VM, Lazarev G, Korolkov N. The solubility of certain transition metals in aluminium. Met Term Obrab Met. 1959;10:48–50.
Drits ME, Kadaner ES, Kuz’mina VL. Solubility of silicon and zirconium in aluminium. Izv Akad Nauk. 1968;1:102–5.
Kuznetsov GM, Barsukov AD, Abas MI. Solubility of Mn, Cr, Ti and Zr in Al in the solid state. Sov Non Ferrous Met Res. 1983;11:47–51.
Murray J, Peruzzi A, Abriata JP. The Al–Zr (aluminum–zirconium) system. J Phase Equilibria. 1992;13:277–91.
Chaudhury ZA, Suryanarayana C. A TEM study of decomposition behavior of a melt-quenched Al–Zr alloy. Metallography. 1994;17:231–52.
Vecchio KS, Williams DB. Convergent beam electron diffraction study of Al3Zr in Al–Zr and Al–Li–Zr alloys. Acta Metall. 1987;35:2959–70.
Dahl W, Gruhl W, Burchard WG, Ibe G, Dumitrecu C. Solidification and precipitation behavior of Al–Zr alloys. II. Precipitation processes in Al–Zr alloys. Z Metallkd. 1977;68:188–94.
Colinet C, Pasturel A. Phase stability and electronic structure in ZrAl3 compound. J Alloys Compd. 2001;319:154–61.
Saunders N, Rivlin VG. Thermodynamic characterization of Al–Cr, Al–Zr and Al–Cr–Zr alloy systems. Mater Sci Technol. 1986;2:521–7.
Saunders N. Calculated stable and metastable phase equilibria in Al–Li–Zr alloys. Z Metallkd. 1989;80(12):894–903.
Wang T, Jin Z, Zhao JC. Thermodynamic assessment of the Al–Zr binary system. J Phase Equilibria. 2001;22:544–51.
Kematik RJ, Franzen HF. Thermodynamic study of the zirconium–aluminium system. J Solid State Chem. 1984;54:226–34.
Brauer G. Crystal structure of intermetallic alloys of aluminium with titanium, zirconium, thorium, niobium and tantalum. Naturwissenschaften. 1938;26:710.
Wilson CG. The crystal structure of ZrAl2. Acta Crystallogr. 1959;12:660–2.
Potzschke M, Schubert K. On the construction of some T4-B3 homologous and quasihomologous systems. II. The Ti–Al, Zr–Al, Hf–Al, Mo–Al and some ternary systems. Z Metallkd. 1962;53:548–61.
Muts N, Gladyshevskii R, Gladyshevskii E. Crystal structures of the compounds PrAl2Si2, Pr3Al4Si6 and PrAlSi2. J Alloys Compd. 2005;402:66–9.
Pisch A, Pasturel A. Heat of dissolution of Nb in liquid Al. Thermochimica Acta. (in press).
Kubaschewski O, Alcock CB, Spencer PJ. Materials thermochemistry. 6th edn. Oxford: Pergamon Press; 1993.
Acknowledgments
The authors greatly acknowledge the financial support by the French National Agency of the Research (ANR) within the framework of the P3N Program (PEPS Project).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janghorban, A., Antoni-Zdziobek, A., Lomello-Tafin, M. et al. Phase equilibria in the aluminium-rich side of the Al–Zr system. J Therm Anal Calorim 114, 1015–1020 (2013). https://doi.org/10.1007/s10973-013-3113-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3113-4