Abstract
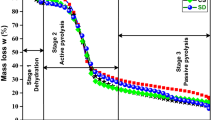
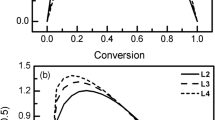
Thermal degradation behavior and kinetics of a biomass waste material, namely walnut shell, were investigated by using a thermogravimetric analyzer. The desired final temperature of 800 °C was achieved at three different heating rates (2, 10, and 15 °C min−1) under nitrogen flow (50 mL min−1). The TG and DTG curves exhibited three distinct zones that can mainly be attributed to removal of water, decomposition of hemicellulose + cellulose, and decomposition of lignin, respectively. The kinetic parameters (activation energy, pre-exponential factor, and reaction order) of active pyrolysis zone were determined by applying Arrhenius, Coats–Redfern, and Horowitz–Metzger methods to TG results. The values of activation energies were found to be between 45.6 and 78.4 kJ mol−1. There was a great agreement between the results of Arrhenius and Coats–Redfern methods while Horowitz–Metzger method yielded relatively higher results. The existence of kinetic compensation effect was evident.








Similar content being viewed by others
References
IEA. World energy outlook. Paris: OECD/IEA; 2008.
Bassam NE. Handbook of energy crops: a complete reference to species, development and applications. UK: Earthscan; 2010.
Karaca F, Bolat E. Coprocessing of a Turkish lignite with a cellulosic waste material 1. The effect of coprocessing on liquefaction yields at different reaction temperatures. Fuel Process Technol. 2000;64:47–55.
Naik S, Goud VV, Rout PK, Jacobson K, Dalai AK. Characterization of Canadian biomass for alternative renewable fuel. Renew Energy. 2010;35:1624–31.
Bridgwater AV, Peacocke GVC. Fast pyrolysis processes for biomass. Renew Sustain Energy Rev. 2000;4:1–73.
Senneca O. Kinetics of pyrolysis, combustion and gasification of three biomass fuels. Fuel Process Technol. 2007;88:87–97.
Bahng M-K, Mukarakate C, Robichaud DJ, Nimlos MR. Current technologies for analysis of biomass thermochemical processing: a review. Anal Chim Acta. 2009;651:117–38.
Yaman S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers Manag. 2004;45:651–71.
García-Ibañez P, Sánchez M, Cabanillas A. Thermogravimetric analysis of olive-oil residue in air atmosphere. Fuel Process Technol. 2006;87(2):103–7.
Sharma A, Rao TR. Kinetics of pyrolysis of rice husk. Bioresour Technol. 1999;67:53–9.
Alvarez VA, Vázquez A. Thermal degradation of cellulose derivatives/starch blends and sisal fibre biocomposites. Polym Degrad Stab. 2004;84:13–21.
Janković B, Adnadević B, Jovanović J. Non-isothermal kinetics of dehydration of equilibrium swollen poly(acrylic acid) hydrogel. J Therm Anal Calorim. 2005;82:7–13.
Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicelluloses, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–8.
Idris SS, Rahman NA, Ismail K, Alias AB, Rashid ZA, Aris MJ. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour Technol. 2010;101:4584–92.
Blasi CD. Modeling chemical and physical processes of wood and biomass pyrolysis. Progr Energy Combust. 2008;34:47–90.
Wu Y-M, Zhao Z-L, Li H-B, He F. Low temperature pyrolysis characteristics of major components of biomass. J Fuel Chem Technol. 2009;37(4):427–32.
Zapata B, Balmesada J, Fragoso-Israel E, Torres-García E. Thermo-kinetics study of orange peel in air. J Therm Anal Calorim. 2009;98:309–15.
Souza BS, Moreira APD, Teixeira AMRF. TG-FTIR coupling to monitor the pyrolysis products from agricultural residues. J Therm Anal Calorim. 2009;97:637–42.
Aboulkas A, El Harfi K, El Bouadili A. Pyrolysis of olive residue/low density polyethylene mixture: part I thermogravimetric kinetics. J Fuel Chem Technol. 2008;36(6):672–8.
Lapuerta M, Hernández JJ, Rodríguez J. Kinetics of devolatilisation of forestry wastes from thermogravimetric analysis. Biomass Bioenergy. 2004;27:385–91.
Ebrahimi-Kahrizsangi R, Abbasi MH. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans Nonferrous Met Soc China. 2008;18:217–21.
Aly AAM, Osman AH, El-Mottaleb MA, Gouda GAH. Thermal stability and kinetic studies of cobalt (II), nickel (II), copper (II), cadmium (II) and mercury (II) complexes derived from n-salicylidene Schiff bases. J Chil Chem Soc. 2009;54(4):349–53.
L’vov BV. Thermal decomposition of solids and melts: new thermochemical approach to the mechanism, kinetics and methodology. Dordrecht, The Netherlands: Springer; 2007.
Acknowledgements
The author is grateful to Işık Yavuz for her valuable help during the analyses. The author would also like to thank Dr. Dilek Duranoğlu and Prof. Dr. Esen Bolat for their continuous support during the studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Açıkalın, K. Thermogravimetric analysis of walnut shell as pyrolysis feedstock. J Therm Anal Calorim 105, 145–150 (2011). https://doi.org/10.1007/s10973-010-1267-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1267-x