Summary
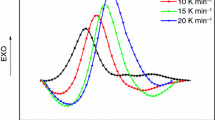
The effect of the concentration of benzyl dimethyl amine as a catalyst on the cure reaction of bisphenol-F based epoxy resin(BPF)/nadic methyl ahydride(NMA) system was studied by differential scanning calorimetery using an isothermal approach over the temperature range 115-145°C. Kinetic parameters of the curing reaction including the reaction order, activation energy and kinetic rate constants were investigated. The results were dependent on the cure temperature and catalyst concentration and proceeded through an autocatalytic kinetic mechanism. The curing kinetic constants and the cure activation energies were obtained by the Arrhenius kinetic model. The suggested kinetic model with a diffusion term was successfully used to describe and predict the cure kinetic of BPF resin compositions as a function of the catalyst content and temperature.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shokrolahi, F., Sadi, M. & Shokrolahi, P. A study on curing kinetic of bisphenol-F using benzyl dimethyl amine by isothermal DSC. J Therm Anal Calorim 82, 151–156 (2005). https://doi.org/10.1007/s10973-005-0856-6
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-0856-6