Abstract
A series of aerogels composed of chitosan and/or silica were fabricated by tuning their feeding ratios. They were characterized by FTIR, thermogravimetric analysis, and X-ray diffraction; pore structures were analyzed by Brunauer–Emmett–Teller (BET) nitrogen sorption and scanning electron microscopy (SEM); adsorption capacities to Congo red were explored as well. The incorporation of silica enhances the thermostabilization of chitosan in gels. And as silica content increases, bulk densities of aerogels decrease gradually, while porosities, pore volumes, and surface areas obtained via BET method increase consequently; as well, porous structure becomes more regular and pore size tends to be smaller that was observed by SEM. The adsorption capacities of chitosan-containing aerogels to Congo red reach as high as about 150 mg/g, much higher than that of pure silica (17 mg/g), demonstrating their potential as a class of novel adsorbent materials.
Graphical Abstract

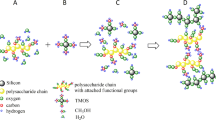
A series of chitosan- and/or silica-based aerogels were fabricated, which were named as C5S0, C4S1, C1S1, C1S4, and C0S5, with different designed CS/SiO2 mass ratios of 100/0, 80/20, 50/50, 20/80, and 0/100, respectively. Their compositions and structures as well as adsorption properties to Congo red were analyzed and compared in detail.





Similar content being viewed by others
References
Pierre AC, Pajonk GM (2002) Chemistry of aerogels and their applications. Chem Rev 102(11):4243–4265. doi:10.1021/Cr0101306
Husing N, Schubert U (1998) Aerogels airy materials: chemistry, structure, and properties. Angew Chem Int Edit 37(1–2):23–45. doi:10.1002/(SICI)1521-3773(19980202)37:1/2<22::AID-ANIE22>3.0.CO;2-I
Jee CSY, Ozguven N, Guo ZX, Evans JRG (2000) Preparation of high porosity metal foams. Metall Mater Trans B 31(6):1345–1352. doi:10.1007/s11663-000-0021-3
Tappan BC, Huynh MH, Hiskey MA, Chavez DE, Luther EP, Mang JT, Son SF (2006) Ultralow-density nanostructured metal foams: combustion synthesis, morphology, and composition. J Am Chem Soc 128(20):6589–6594. doi:10.1021/Ja056550k
Tappan BC, Steiner SA 3rd, Luther EP (2010) Nanoporous metal foams. Angew Chem Int Ed Engl 49(27):4544–4565. doi:10.1002/anie.200902994
Liu H, Yang Q (2011) Facile fabrication of nanoporous Au–Pd bimetallic foams with high catalytic activity for 2-nitrophenol reduction and SERS property. J Mater Chem 21(32):11961–11967. doi:10.1039/c1jm10109a
Caro M, Mook WM, Fu EG, Wang YQ, Sheehan C, Martinez E, Baldwin JK, Caro A (2014) Radiation induced effects on mechanical properties of nanoporous gold foams. Appl Phys Lett 104(23):233109. doi:10.1063/1.4882275
Tang Y, Yeo KL, Chen Y, Yap LW, Xiong W, Cheng WL (2013) Ultralow-density copper nanowire aerogel monoliths with tunable mechanical and electrical properties. J Mater Chem A 1(23):6723–6726. doi:10.1039/C3ta10969k
Ruben GC, Pekala RW (1995) High-resolution transmission electron-microscopy of the nanostructure of melamine-formaldehyde aerogels. J Non-Cryst Solids 186:219–231. doi:10.1016/0022-3093(95)00082-8
Pekala RW (1989) Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci 24(9):3221–3227. doi:10.1007/Bf01139044
Wu D, Fu R (2006) Synthesis of organic and carbon aerogels from phenol–furfural by two-step polymerization. Microporous Mesoporous Mater 96(1):115–120. doi:10.1016/j.micromeso.2006.06.022
Long D, Zhang J, Yang J, Hu Z, Cheng G, Liu X, Zhang R, Zhan L, Qiao W, Ling L (2008) Chemical state of nitrogen in carbon aerogels issued from phenol–melamine–formaldehyde gels. Carbon 46(9):1259–1262. doi:10.1016/j.carbon.2008.04.022
Jirglova H, Perez-Cadenas AF, Maldonado-Hodar FJ (2009) Synthesis and properties of phloroglucinol-phenol-formaldehyde carbon aerogels and xerogels. Langmuir 25(4):2461–2466. doi:10.1021/la803200b
White RJ, Budarin V, Luque R, Clark JH, Macquarrie DJ (2009) Tuneable porous carbonaceous materials from renewable resources. Chem Soc Rev 38(12):3401–3418. doi:10.1039/b822668g
Chang X, Chen D, Jiao X (2010) Starch-derived carbon aerogels with high-performance for sorption of cationic dyes. Polymer 51(16):3801–3807. doi:10.1016/j.polymer.2010.06.018
Tsioptsias C, Michailof C, Stauropoulos G, Panayiotou C (2009) Chitin and carbon aerogels from chitin alcogels. Carbohydr Polym 76(4):535–540. doi:10.1016/j.carbpol.2008.11.018
Quignard F, Valentin R, Di Renzo F (2008) Aerogel materials from marine polysaccharides. New J Chem 32(8):1300–1310. doi:10.1039/B808218a
García-González CA, Alnaief M, Smirnova I (2011) Polysaccharide-based aerogels—promising biodegradable carriers for drug delivery systems. Carbohydr Polym 86(4):1425–1438. doi:10.1016/j.carbpol.2011.06.066
Nardecchia S, Carriazo D, Ferrer ML, Gutiérrez MC, del Monte F (2013) Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: synthesis and applications. Chem Soc Rev 42(2):794–830. doi:10.1039/c2cs35353a
Nadargi DY, Rao AV (2009) Methyltriethoxysilane: new precursor for synthesizing silica aerogels. J Alloy Compd 467(1–2):397–404. doi:10.1016/j.jallcom.2007.12.019
Harreld JH, Ebina T, Tsubo N, Stucky G (2002) Manipulation of pore size distributions in silica and ormosil gels dried under ambient pressure conditions. J Non-Cryst Solids 298(2–3):241–251. doi:10.1016/S0022-3093(01)01051-1
Leventis N, Sotiriou-Leventis C, Zhang GH, Rawashdeh AMM (2002) Nanoengineering strong silica aerogels. Nano Lett 2(9):957–960. doi:10.1021/Nl025690e
Nguyen BN, Meador MA, Tousley ME, Shonkwiler B, McCorkle L, Scheiman DA, Palczer A (2009) Tailoring elastic properties of silica aerogels cross-linked with polystyrene. ACS Appl Mater Interfaces 1(3):621–630. doi:10.1021/am8001617
Meador MAB, Scherzer CM, Vivod SL, Quade D, Nguyen BN (2010) Epoxy reinforced aerogels made using a streamlined process. ACS Appl Mater Interfaces 2(7):2162–2168. doi:10.1021/Am100422x
Wei TY, Lu SY, Chang YC (2008) Transparent, hydrophobic composite aerogels with high mechanical strength and low high-temperature thermal conductivities. J Phys Chem B 112(38):11881–11886. doi:10.1021/jp804855v
Sanli D, Erkey C (2013) Monolithic composites of silica aerogels by reactive supercritical deposition of hydroxy-terminated poly(dimethylsiloxane). ACS Appl Mater Interfaces 5(22):11708–11717. doi:10.1021/am403200d
Litschauer M, Neouze M-A, Haimer E, Henniges U, Potthast A, Rosenau T, Liebner F (2010) Silica modified cellulosic aerogels. Cellulose 18(1):143–149. doi:10.1007/s10570-010-9459-x
Cai J, Liu S, Feng J, Kimura S, Wada M, Kuga S, Zhang L (2012) Cellulose-silica nanocomposite aerogels by in situ formation of silica in cellulose gel. Angew Chem Int Ed Engl 51(9):2076–2079. doi:10.1002/anie.201105730
Sai HZ, Xing L, Xiang JH, Cui LJ, Jiao JB, Zhao CL, Li ZY, Li F (2013) Flexible aerogels based on an interpenetrating network of bacterial cellulose and silica by a non-supercritical drying process. J Mater Chem A 1(27):7963–7970. doi:10.1039/C3ta11198a
Shi J, Lu L, Guo W, Zhang J, Cao Y (2013) Heat insulation performance, mechanics and hydrophobic modification of cellulose-SiO2 composite aerogels. Carbohydr Polym 98(1):282–289. doi:10.1016/j.carbpol.2013.05.082
Yao CJ, Liu X, Risen WM (2011) Biopolymer-containing aerogels: chitosan-silica hybrid aerogels. Aerogels Handbook. Springer, New York. doi:10.1007/978-1-4419-7589-8_18
Hu X, Littrel K, Ji S, Pickles DG Jr, Risen WM (2001) Characterization of silica–polymer aerogel composites by small-angle neutron scattering and transmission electron microscopy. J Non-Cryst Solids 288(1):184–190. doi:10.1016/S0022-3093(01)00625-1
Ayers MR, Hunt AJ (2001) Synthesis and properties of chitosan-silica hybrid aerogels. J Non-Cryst Solids 285(1–3):123–127. doi:10.1016/S0022-3093(01)00442-2
Wang YC, Lin MC, Wang DM, Hsieh HJ (2003) Fabrication of a novel porous PGA-chitosan hybrid matrix for tissue engineering. Biomaterials 24(6):1047–1057. doi:10.1016/s0142-9612(02)00434-9
Liu X, Zhu Y, Yao C, Risen WM (2003) Photo-formed metal nanoparticle arrays in monolithic silica-biopolymer aerogels. MRS Online Proc Libr 788:13–24. doi:10.1557/PROC-788-L2.1
Ogawa T, Watanabe J, Oshima Y (2008) Catalyst-free synthesis of polyorganosiloxanes by high temperature and pressure water. J Supercrit Fluids 45(1):80–87. doi:10.1016/j.supflu.2007.11.010
Pinon-Segundo E, Ganem-Quintanar A, Flores-Flores JO, Saniger-Blesa JM, Urban-Morlan MZ, Mendoza-Romero L, Nava-Arzaluz MG, Quintanar-Guerrero D (2008) Evaluation of SiO2 sonogels, prepared by a new catalyst-free method, as drug delivery system. Drug Deliv 15(6):399–407. doi:10.1080/10717540802039162
Morales-Saavedra OG, Rivera E, Flores-Flores JO, Castaneda R, Banuelos JG, Saniger JM (2007) Preparation and optical characterization of catalyst free SiO2 sonogel hybrid materials. J Sol-Gel Sci Technol 41(3):277–289. doi:10.1007/s10971-006-9006-2
Yang YM, Wang JW, Tan RX (2004) Immobilization of glucose oxidase on chitosan–SiO2 gel. Enzyme Microbial Technol 34(2):126–131. doi:10.1016/j.enzmictec.2003.09.007
Al-Sagheer F, Muslim S (2010) Thermal and mechanical properties of chitosan/SiO2 hybrid composites. J Nanomater 2010:1–7. doi:10.1155/2010/490679
Rashidova SS, Shakarova DS, Ruzimuradov ON, Satubaldieva DT, Zalyalieva SV, Shpigun OA, Varlamov VP, Kabulov BD (2004) Bionanocompositional chitosan-silica sorbent for liquid chromatography. J Chromatogr B 800(1–2):49–53. doi:10.1016/j.jchromb.2003.10.015
Cestari AR, Vieira EF, Pinto AA, Lopes EC (2005) Multistep adsorption of anionic dyes on silica/chitosan hybrid. 1. Comparative kinetic data from liquid- and solid-phase models. J Colloid Interface Sci 292(2):363–372. doi:10.1016/j.jcis.2005.05.096
Copello GJ, Mebert AM, Raineri M, Pesenti MP, Diaz LE (2011) Removal of dyes from water using chitosan hydrogel/SiO2 and chitin hydrogel/SiO2 hybrid materials obtained by the sol–gel method. J Hazard Mater 186(1):932–939. doi:10.1016/j.jhazmat.2010.11.097
Zulfikar MA, Wahyuningrum D, Lestari S (2013) Adsorption of lignosulfonate compound from aqueous solution onto chitosan-silica beads. Sep Sci Technol 48(9):1391–1401. doi:10.1080/01496395.2012.728275
Wang L, Wang A (2007) Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J Hazard Mater 147(3):979–985. doi:10.1016/j.jhazmat.2007.01.145
Du Q, Sun J, Li Y, Yang X, Wang X, Wang Z, Xia L (2014) Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem Eng J 245:99–106. doi:10.1016/j.cej.2014.02.006
Luk CJ, Yip J, Yuen CM, Kan C, Lam K (2014) A comprehensive study on adsorption behaviour of direct, reactive and acid dyes on crosslinked and non-crosslinked chitosan beads. J Fiber Bioeng Inform 7(1):35–52. doi:10.3993/jfbi03201404
Peng Q, Liu M, Zheng J, Zhou C (2015) Adsorption of dyes in aqueous solutions by chitosan–halloysite nanotubes composite hydrogel beads. Microporous Mesoporous Mater 201:190–201. doi:10.1016/j.micromeso.2014.09.003
Acknowledgments
We would like to thank the anonymous reviewers and the editor for their constructive comments. This work was financially supported by National Natural Science Foundation of China (21004005), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (20120932003).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Zhou, Q., Song, D. et al. Chitosan–silica composite aerogels: preparation, characterization and Congo red adsorption. J Sol-Gel Sci Technol 76, 501–509 (2015). https://doi.org/10.1007/s10971-015-3800-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3800-7