Abstract
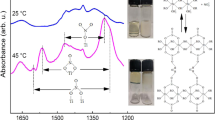
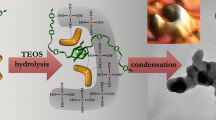
FTIR has been used to follow the evolution of a sol–gel preparation accomplished with two acid/acid steps. The reaction of methyltrimethoxysilane mixed with colloidal silica was taken as an example, aiming at proving the use of IR spectroscopy for the determination of reaction aspects like the kinetic behaviour that is essential for the scale-up of the application. The steps were carried out for 60 min each and samples were taken in each step at variable time intervals. For the spectroscopy analysis the dispersion samples were deposited on a spinning disc of KBr or ZnSe obtaining thin hybrid organic/inorganic films. The signals at 950 cm−1 and 1,000–1,100 cm−1 related to the hydrolysis and condensation reaction, respectively were recorded at variable time and their intensity normalised to the 1,273 cm−1band of Si-CH3 group not involved in the hydrolysis nor condensation reaction. The effects of pH and temperature have been investigated showing that reliable effective data on the reaction extent of SiO2-doped sol–gel dispersions can be obtained by the FTIR spectroscopy. The data have been supported and interpreted also by SEM observations. The reactions degree of the (poly)condensation sharply increases after the addition of more alkoxysilane. Short reaction times can be designed that could be compatible with industrial production.








Similar content being viewed by others
References
Mackenzie JD (2003) Sol–Gel research—achievements since 1981 and prospects for the future. J Sol–Gel Sci Technol 26:23–27
Aegerter MA, Almeida R, Soutar A, Tadanaga K, Yang H, Watanabe T (2008) Coatings made by sol–gel and chemical nanotechnology. J Sol–Gel Sci Technol 47:203–236
Schottner G, Rose K, Posset U (2003) Scratch and abrasion resistant coatings on plastic lenses—state of the art, current developments and perspectives. J Sol–Gel Sci Technol 27:71–79
Gallardo J, Duran A, Garcia I, Celis JP, Arenas MA, Conde A (2003) Effect of sintering temperature on the corrosion and wear behaviour of protective SiO2-based Sol–gel coatings. J Sol–Gel Sci Technol 27:175183
Bestetti M, Forno AD, Cavallotti PL, Gronchi P, Barlassina F (2010) Anodic oxidation and sol–gel coatings for corrosion and wear protection of AM60B alloy. Trans Inst Met Fin 88:57–62
Uhlmann DR, Teowee G (1998) Sol gel science and technology: current state and future prospects. J Sol–Gel Sci Technol 13:153–162
Rao AV, Bhagat S (2004) Synthesis and physical properties of TEOS-based silica aerogels prepared by two step (acid–base) sol–gel process. Sol State Sci 6:945–952
Nadargi DY, Latthe SS, Rao AV (2009) Effect of post-treatment (gel aging) on the properties of methyltrimethoxysilane based silica aerogels prepared by two-step sol–gel process. J Sol–Gel Sci. Technol. 49:53–59
Kim SM, Chakrabarti K, Oh EO, Whang CM (2003) Effects of pH during the base catalyzed reaction of two-step acid/base catalyzed process on the microstructures and physical properties of poly(dimethylsiloxane) modified silica xerogels. J Sol–Gel Sci Technol 27:149–155
Parvathy-Rao A, Pajonk GM, Rao∗ AV (2005) Effect of preparation conditions on the physical and hydrophobic properties of two step processed ambient pressure dried silica aerogels. J Mat Sci 40:3481–3489
Dong H, Brook MA, Brennan JD (2005) A new route to monolithic methylsilsesquioxanes: gelation behavior of methyltrimethoxysilane and morphology of resulting methylsilsesquioxanes under one-step and two-step processing. Chem Mat 17:2807–2816
Wang J, Wu G, Shen J, Yang T, Zhang Q, Zhou B, Deng Z (2000) Scratch-resistant improvement of Sol–Gel derived nano-porous silica films. J Sol–Gel Sci Technol 18:219–224
Daniels MW, Sefcik J, Francis LF, McCormick AV (1999) Reactions of a trifunctional silane coupling agent in the presence of colloidal silica sols in polar media. J Coll Interf Sci 219:351–356
Lee MS, Jo NJ (2002) Coating of methyltriethoxysilane—modified colloidal silica on polymer substrates for abrasion resistance. J Sol–Gel Sci Technol 24:175–180
de Lange RSA, Hekkink JHA, Keizer K, Burggraaf AJ (1995) Polymeric-silica-based sols for membrane modification applications: sol–gel synthesis and characterization with SAXS. J Non-Cryst Solids 191:1–16
Schmidt H, Scholze H, Kaiser A (1984) Principles of hydrolysis and condensation reaction of alkoxysilanes. J Non-CrystSolids 63:1
Pouxviel JC, Boilot JP, Beloeil JC, Lallemand JY (1987) NMR study of the sol/gel polymerization. J Non-Cryst Solids 89:345
Kline AA, Rogers TN, Mullins ME, Cornilsen BC, Solokov LM (1994) Sol–gel kinetics for the synthesis of multi-component glass materials. J Sol–Gel Sci Technol 2:269–272
Jiang H, Zheng Z, Wang X (2008) Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vibr Spectrosc 46:1–7
Zhang Z, Gorman BP, Dong H, Orozco-Teran RA, Mueller DW, Reidy RF (2003) Investigation of polymerization and cyclization of dimethyldiethoxysilane by 29Si NMR and FTIR. J Sol–Gel Sci Technol 28:159–165
Deng Q, Moore RB, Mauritz KA (1995) Novel nafion/ORMISIL hybrids via in situ sol–gel reactions. 1. Probe of ormisil phase nanostructures by infrared spectroscopy. Chem Mat 7:2259–2268
Lenza RFS, Vasconcelos WL (2001) Structural evolution of silica sols modified with formamide. Mat Res Inn 4:175–179
Matsumoto T, Takayama Y, Wada NHO, Kojima K, Yamada H, Wakabayashi H (2003) Acid-free synthesis of poly-organo-siloxane spherical particles using a W/O emulsion. J Mater Chem 13:1764–1770
Tejedor-Tejedor MI, Paredes L, Anderson MA (1998) Evaluation of ATR-FTIR spectroscopy as an “in situ” tools for following the hydrolysis and condensation of alkoxysilanes under rich H2O conditions. Chem Mater 10:3410
Brinker CJ, Scherer GW (1990) Sol–Gel science: the physics and chemistry of sol–gel processing. Academic Press, San Diego
Aguiar H, Serra J, González P, León B (2009) Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J Non-Cryst Solids 355:475–487
Voronkov MG, Mileshkevich VP, Yuzhelevski YA (1978) Siloxane bond. Consultants Bureau, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amoriello, S., Bianco, A., Eusebio, L. et al. Evolution of two acid steps sol–gel phases by FTIR. J Sol-Gel Sci Technol 58, 209–217 (2011). https://doi.org/10.1007/s10971-010-2379-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-010-2379-2