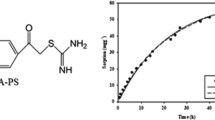
Abstract
Fumarated polystyrene microspheres were prepared using emulsion polymerization technique. Different instrumental techniques such as elemental analysis, SEM and FTIR were employed for full characterization of the synthetic resin. Different parameters such as pH, time and initial metal ions concentration were examined to evaluate the optimum conditions for U(VI) and Nd(III) ions sorption. For both metal ions, the sorption process fitted well with pseudo-second order kinetic model and Langmuir isotherm model. The maximum adsorption capacities were found to be 83.11 mg U g−1 and 39.68 mg Nd g−1. The loaded sorbent was regenerated using 0.5 M HNO3.






Similar content being viewed by others
References
Kumar A, Singhal RK, Rout S, Narayanan U, Karpe R, Ravi PM (2013) Adsorption and kinetic behavior of uranium and thorium in seawater-sediment system. J Radioanal Nucl Chem 295:649–656
Elsalamouny AR, Desouky OA, Mohamed SA, Galhoum AA, Guibal E (2017) Uranium and neodymium biosorption using novel chelating polysaccharide. Int J Biol Macromol 104:963–968
Zhang X, Ding C, Liu H, Liu L, Zhao C (2011) Protective effects of ion-imprinted Chitooligosaccharides as uranium-specific chelating agents against the cytotoxicity of depleted uranium in human kidney cells. Toxicology 286:75–84
Zou Y, Wang X, Wu F, Yu S, Hu Y, Song W, Liu Y, Wang H, Hayat T, Wang X (2017) Controllable synthesis of Ca–Mg–Al layered double hydroxides and calcined layered double oxides for the efficient removal of U(VI) from wastewater solutions. ACS Sustain Chem Eng. 5:1173–1185
Bayramoglu G, Arica MY (2016) MCM-41 silica particles grafted with polyacrylonitrile: modification into amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous Mesoporous Mater 226:117–124
Arica MY, Bayramoglu G (2016) Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. J Radioanal Nucl Chem 310:711–724
Meinrath G (1998) Aquatic chemistry of uranium. Geoscience 1:1–101
Rao TP, Metilda P, Gladis JM (2006) Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination—an overview. Talanta 68:1047–1064
Zou Y, Liu Y, Wang X, Sheng G, Wang S, Ai Y, Ji Y, Liu Y, Hayat T, Wang X (2017) Glycerol-modified binary layered double hydroxide nanocomposites for uranium immobilization via EXAFS technique and DFT theoretical calculation. ACS Sustain Chem Eng 5:3583–3595
Bayramoglu G, Arica MY (2017) Polyethylenimine and tris(2-aminoethyl)amine modified p(GA–EGMA) microbeads for sorption of uranium ions: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 312:293–303
Galhoum AA, Mahfouz MG, Abdel-Rehem ST, Gomaa NA, Atia AA, Vincent T, Guibal E (2015) Diethylenetriamine-functionalized chitosan magnetic nanobased particles for the sorption of rare earth metal ions [Nd(III), Dy(III) and Yb(III)]. Cellulose 22:2589–2605
Xu T, Peng H (2009) Formation cause, composition analysis and comprehensive utilization of rare earth solid wastes. J Rare Earths 27:1096–1102
Zhou J, Duan W, Zhou X, Zhang C (2007) Application of annular centrifugal contactors in the extraction flowsheet for producing high purity yttrium. Hydrometallurgy 85:154–162
Diniz V, Volesky B (2005) Biosorption of La, Eu and Yb using sargassum biomass. Water Res 39:239–247
Martins TS, Isolani PC (2005) Rare earths: industrial and biological applications. Quim Nova 28:111–117
Donia AM, Atia AA, Daher AM, Desouky OA, Elshehy EA (2011) Synthesis of amine/thiol magnetic resin and study of its interaction with Zr(IV) and Hf(IV) ions in their aqueous solutions. J Dispers Sci Technol 32:634–641
Roosen J, Binnemans K (2014) Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J Mater Chem A 2:1530–1540
Wang H, Ma L, Cao K, Geng J, Liu J, Song Q, Yang X, Li S (2012) Selective solid phase extraction of uranium by salicylideneimine-functionalized hydrothermal carbon. J Hazard Mater 229:321–330
Zou Y, Wang X, Khan A, Wang P, Liu Y, Alsaedi A, Hayat T, Wang X (2016) Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review. Environ Sci Technol 50:7290–7304
Erkaya IA, Arica MY, Akbulut A, Bayramoglu G (2014) Biosorption of uranium(VI) by free and entrapped Chlamydomonas reinhardtii: kinetic, equilibrium and thermodynamic studies. J Radioanal Nucl Chem 299:1993–2003
Bayramoglu G, Akbulut A, Arica MY (2015) Study of polyethyleneimine and amidoxime functionalized hybrid biomass of Spirulina (Arthrospira) platensis for adsorption of uranium (VI) ion. Environ Sci Pollut Res 22:17998–18010
Bayramoglu G, Arica MY (2016) Amidoxime functionalized Trametes trogii pellets for removal of uranium (VI) from aqueous medium. J Radioanal Nucl Chem 307:373–384
Bhattarai S, Kim J, Yun Y, Lee Y (2016) Preparation of polyaniline-coated polystyrene nanoparticles for the sorption of silver ions. React Funct Polym 105:52–59
Davarpanaha M, Ahmadpoura A, Rohani-Bastamia T, Dabirb H (2015) Synthesis and application of diethanolamine-functionalized polystyrene as a new sorbent for the removal of p-toluenesulfonic acid from aqueous solution. J Ind Eng Chem 30:281–288
Kadous A, Didi MA, Villemin D (2010) A new sorbent for uranium extraction: ethylenediamino tris(methylenephosphonic) acid grafted on polystyrene resin. J Radioanal Nucl Chem 284:431–438
Mahfouz MG, Killa HM, Sheta ME, Moustafa AH, Tolba AA (2014) Synthesis, characterization, and application of polystyrene adsorbents containing tri-n-butylphosphate for solid-phase extraction of uranium (VI) from aqueous nitrate solutions. J Radioanal Nucl Chem 301:739–749
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 53:97–110
Song W, Wang X, Wang Q, Shao D, Wang X (2015) Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys Chem Chem Phys 17:398–406
Cheng W, Ding C, Wu Q, Wang X, Sun Y, Shi W, Hayat T, Alsaedi A, Chaicf Z, Wang X (2017) Mutual effect of U(VI) and Sr(II) on grapheme oxides: evidence from EXAFS and theoretical calculations. Environ Sci 4:1124–1131
Sun Y, Yang S, Chen Y, Ding C, Cheng W, Wang X (2015) Adsorption and desorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study. Environ Sci Technol 49:4255–4262
Sun Y, Wu Z, Wang X, Ding C, Cheng W, Yu S, Wang X (2016) Macroscopic and microscopic investigation of U(VI) and Eu(III) adsorption on bacterium-derived carbon nanofibers. Environ Sci Technol 50:4459–4467
Sun Y, Wang X, Ai Y, Yu Z, Huang W, Chen C, Hayat T, Alsaedi A, Wang X (2017) Interaction of sulfonated graphene oxide with U(VI) studied by spectroscopic analysis and theoretical calculations. Chem Eng J 310:292–299
Wang X, Fan Q, Yu S, Chen Z, Ai Y, Sun Y, Hobiny A, Alsaedi A, Wang X (2016) High sorption of U(VI) on graphene oxides studied by batch experimental and theoretical calculations. Chem Eng J 287:448–455
Yin L, Wang P, Wen T, Yu S, Wang X, Hayat T, Alsaedi A, Wang X (2017) Synthesis of layered titanate nanowires at low temperature and their application in efficient removal of U(VI). Environ Pollut 226:125–134
Drits VA, Silvester E, Gorshkov AI, Manceau A (1997) Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite: I. Results from X-ray diffraction and selected-area electron diffraction. Am Mineral 82:946–961
Peacock CL, Sherman DM (2007) Sorption of Ni by birnessite: equilibrium controls on Ni in seawater. Chem Geol 238:94–106
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Vetenskapsakademiens Handlingar 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Elwakeel KZ, Abd El-Ghaffar MA, El-Kousy SM, El-Shorbagy HG (2012) Synthesis of new ammonium chitosan derivatives and their application for dye removal from aqueous media. Chem Eng J 203:458–468
Crank J (1975) The mathematics of diffusion, 2nd edn. Oxford University Press, London
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Ahmed IM, Gamal R, Helal AA, Abo-Elenein SA, Helal AA (2016) Kinetic sorption study of cerium (IV) on magnetite nanoparticles. Part Sci Technol. doi:10.1080/02726351.2016.1192572
Das D, Sureshkumar MK, Koley S, Mithal N, Pillai CGS (2010) Sorption of uranium on magnetite nanoparticles. J Radioanal Nucl Chem 285:447–454
Elsalamouny AR, Desouky OA, Mohamed SA, Galhoum AA (2017) Evaluation of adsorption behavior for U(VI) and Th(IV) ions onto solidified Mannich type material. J Dispers Sci Technol 38:860–865
Stopa LCB, Yamaura M (2010) Uranium removal by chitosan impregnated with magnetite nanoparticles: adsorption and desorption. Int J Nucl Energy Sci Technol 5:283–289
Wang JS, Peng RT, Yang JH, Liu YC, Hu XJ (2011) Preparation of ethylenediamine-modified magnetic chitosan complex for adsorption of uranyl ions. Carbohydr Polym 84:1169–1175
Rahmati A, Ghaemi A, Samadfam M (2012) Kinetic and thermodynamic studies of uranium(VI) adsorption using Amberlite IRA-910 resin. Ann Nucl Energy 39:42–48
Kozhevnikova NM, Tsybikova NL (2008) Sorption of neodymium(III) ions by natural mordenite-containing tuff. Russ J Appl Chem 81:42–45
Vlachou A, Symeopoulos BD, Koutinas AA (2009) A comparative study of neodymium sorption by yeast cells. Radiochim Acta 97:437–441
Krishna PG, Gladis JM, Rao TP, Naidu GR (2005) Selective recognition of neodymium(III) using ion imprinted polymer particles. J Mol Recognit 18:109–116
Tak RK, Gupta BD, Sobhash PD, Sharma A, Mathur SP (1990) Some rare earth chelates on N-thioacetyl-N-phenylhydroxylamine. Asian J Chem 2:132–135
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elsalamouny, A.R., Desouky, O.A., Mohamed, S.A. et al. Evaluation of adsorption behavior for U(VI) and Nd(III) ions onto fumarated polystyrene microspheres. J Radioanal Nucl Chem 314, 429–437 (2017). https://doi.org/10.1007/s10967-017-5389-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5389-5