Abstract
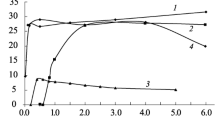
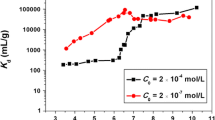
The sorption–desorption of Eu(III) on attapulgite was studied using batch technique. The sorption of Eu(III) on attapulgite was strongly dependent on pH and ionic strength at low pH, and independent of ionic strength at high pH. The interaction was dominated by outer-sphere surface complexation/ion exchange at low pH, and by inner-sphere surface complexation at high pH. The irreversible sorption–desorption curves also suggested the formation of strong surface complexes. The high sorption capacity of attapulgite suggested that the attapulgite is a suitable material for the efficient elimination of Eu(III) from aqueous solutions in nuclear wastewater management.









Similar content being viewed by others
References
Tan XL, Ren XM, Chen CL, Wang XK (2014) Analytical approaches to the speciation of lanthanides on solid–water interfaces. Trend Anal Chem (TrAC) 61:107–132
Yang S, Sheng G, Tan X, Hu J, Du J, Montavon G, Wang X (2011) Determination of Ni(II) sorption mechanisms on mordenite surfaces: a combined macroscopic and microscopic approach. Geochim Cosmochim Acta 75:6520–6534
Hu R, Shao D, Wang X (2014) Graphene oxide/polypyrrole composites for highly selective enrichment of U(VI) from aqueous solutions. Polym Chem 5:6207–6215
Wang X, Chen C, Hu W, Ding A, Xu D, Zhou X (2005) Sorption of 243Am(III) to multi-wall carbon nanotubes. Environ Sci Technol 39:2856–2860
Hurel C, Marmier N (2010) Sorption of europium on a MX-80 bentonite sample: experimental and modelling results. J Radioanal Nucl Chem 284:225–230
Lu SS, Xu JZ, Zhang CC, Niu ZW (2011) Adsorption and desorption of radionuclide europium(III) on multiwalled carbon nanotubes studied by batch techniques. J Radioanal Nucl Chem 287:893–898
Shao DD, Fan QH, Li JX, Niu ZW, Wu WS, Chen YX, Wang XK (2009) Removal of Eu(III) from aqueous solution using ZSM-5 zeolite. Microporous Mesoporous Mater 123:1–9
Hu J, Xie Z, He B, Sheng G, Chen C, Li J, Chen Y, Wang X (2010) Sorption of Eu(III) on GMZ bentonite in the absence/presence of humic acid studied by batch and XAFS techniques. Sci China B 53:1420–1428
Das DK, Pathak PN, Kumar S, Manchanda VK (2009) Sorption behavior of Am 3 + on suspended pyrite. J Radioanal Nucl Chem 281:449–455
Palagyi S, Vodickova H, Landa J, Palagyiova J, Laciok A (2009) Migration and sorption of Cs-137 and Eu-152, Eu-154 in crushed crystalline rocks under dynamic conditions. J Radioanal Nucl Chem 279:431–441
Yang S, Zong P, Ren X, Wang Q, Wang X (2012) Rapid and high-efficient preconcentration of Eu(III) by core-shell structured humic acid@Fe3O4 magnetic nanoparticles. ACS Appl Mater Interface 4:6891–6900
Yang S, Sheng G, Montavon G, Guo Z, Tan X, Grambow B, Wang X (2013) Investigation of Eu(III) immobilization on γ-Al2O3 surfaces by combining batch technique and EXAFS analysis: role of contact time and humic acid. Geochim Cosmochim Acta 121:84–104
Rabung Th, Geckeis H, Kim J, Beck HP (1998) The influence of anionic ligands on the sorption behavior of Eu(III) on natural hematite. Radiochim Acta 82:243–248
Sheng G, Yang S, Li Y, Gao X, Huang Y, Hu J, Wang X (2014) Retention mechanisms and microstructure of Eu(III) on manganese dioxide studied by batch and high resolution EXAFS technique. Radiochim Acta 102:155–167
Janot N, Benedetti MF, Reille PE (2011) Colloidal alpha–Al2O3, Europium (III) and humic substances interactions: a macroscopic and spectroscopic study. Environ Sci Technol 45:3224–3230
Tan XL, Fan QH, Wang XK, Grambow B (2009) Eu(III) sorption to TiO2 (anatase and rutile): batch, XPS, and EXAFS study. Environ Sci Technol 43:3115–3121
Rabung T, Pierret MC, Bauer A, Geckeis H, Bradbury MH, Baeyens B (2005) Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 1: batch sorption and time-resolved laser fluorescence spectroscopy experiments. Geochim Cosmochim Acta 69:5393–5402
Wang X, Zhou X, Du J, Hu W, Chen C, Chen Y (2006) Using of chelating resin to study the kinetic desorption of Eu(III) from humic acid–Al2O3 colloid surfaces. Surf Sci 600:478–483
Wang X, Sun Y, Wang X (2015) Interaction mechanism of Eu(III) with MX-80 bentonite studied by batch, TRLFS and kinetic desorption techniques. Chem Eng J 264:570–576
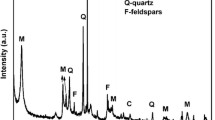
Fan Q, Tan X, Li J, Wang X, Wu W, Montavon G (2009) Sorption of Eu(III) on attapulgite studied by batch, XPS and EXAFS techniques. Environ Sci Technol 43:5776–5782
Fan Q, Shao D, Wu W, Wang X (2009) Effect of pH, ionic strength, temperature and humic substances on the sorption of Ni(II) to Na-attapulgite. Chem Eng J 150:188–195
Chen C, Yang X, Wei J, Tan X, Wang X (2013) Eu(III) uptake on rectorite in the presence of humic acid: a macroscopic and spectroscopic study. J Colloid Interface Sci 393:249–256
Montavon G, Rabung T, Geckeis H, Grambow B (2004) Interaction of Eu(III)/Cm(III) with alumina-bound poly(acrylic acid): sorption, desorption, and spectroscopic studies. Environ Sci Technol 38:4312–4318
Ren X, Li J, Tan X, Shi W, Chen C, Shao D, Wen T, Wang L, Zhao G, Sheng G, Wang X (2014) Impact of Al2O3 on the aggregation and deposition of graphene oxide. Environ Sci Technol 48:5493–5500
Giustetto R, Xamena FXL, Ricchiardi G, Bordiga S, Damin A, Gobetto R, Chierotti MR (2005) Maya blue: a computational and spectroscope study. J Phys Chem B 109:19360–19368
Li A, Wang A (2005) Synthesis and properties of clay-based superabsorbent composite. Eur Polym J 41:1630–1637
Wu WS, Fan QH, Xu JZ, Niu ZW, Lu SS (2007) Sorption–desorption of Th(IV) on attapulgite: effects of pH, ionic strength and temperature. Appl Radiat Isot 65:1108–1114
Fan QH, Zhang ML, Zhang YY, Ding KF, Yang ZQ, Wu WS (2010) Sorption of Eu(III) and Am(III) on attapulgite: effect of pH, ionic strength and fulvic acid. Radiochim Acta 98:19–25
Sheng G, Yang S, Sheng J, Hu J, Tan X, Wang X (2011) Macroscopic and microscopic investigation of Ni(II) sequestration on diatomite by batch, XPS and EXAFS techniques. Environ Sci Technol 45:7718–7726
Lee S, Anderson PR, Bunker GB, Karanfil C (2004) EXAFS study of Zn sorption mechanisms on montmorillonite. Environ Sci Technol 38:5426–5432
Kumar S, Kasar SU, Bajpai RK, Kaushik CP, Guin R, Das SK, Tomar BS (2014) Kinetics of Pu(IV) sorption by smectite-rich natural clay. J Radioanal Nucl Chem 300:45–59
Sun Y, Li J, Wang X (2014) The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques. Geochim Cosmochim Acta 140:621–643
Chen C, Wang X, Nagatsu M (2009) Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ Sci Technol 43:2362–2367
Sun Y, Wang Q, Chen C, Tan X, Wang X (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6207
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide supported polyaniline. Environ Sci Technol 47:9904–9910
Takahashi Y, Kimura T, Kato Y, Minai Y (1999) Speciation of europium(III) sorbed on a montmorillonite surface in the presence of polycarboxylic acid by laser-induced fluorence spectroscopy. Environ Sci Technol 33:4016–4021
Montavon G, Hennig C, Janvier C, Grambow B (2006) Comparison of complexed species of Eu in alumina-bound and free polyacrylic acid: a spectroscopic study. J Colloid Inteface Sci 300:482–490
Tan X, Wang X, Geckeis H, Rabung T (2008) Sorption of Eu(III) on humic acid or fulvic acid bound to alumina studied by SEM-EDS, XPS, TRLFS and batch techniques. Environ Sci Technol 42:6532–6537
Yang ST, Zong PF, Sheng GD, Ren XM, Huang YY, Wang XK (2014) New insight into Eu(III) sorption mechanism at alumina/water interface by batch technique and EXAFS analysis. Radiochim Acta 102:143–153
Janot N, Benedetti MF, Reiller PE (2011) Colloidal α-Al2O3, europium(III) and humic substances interactions: a macroscopic and spectroscopic study. Environ Sci Technol 45:3224–3230
Sun Y, Chen C, Tan X, Shao D, Li J, Zhao G, Yang S, Wang Q, Wang X (2012) Enhanced adsorption of Eu(III) on mesoporous Al2O3/expanded graphite composites investigated by macroscopic and microscopic techniques. Dalton Trans 41:13388–13394
Song W, Wang X, Wang Q, Shao D, Wang X (2015) Plasma induced grafting polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys Chem Chem Phys 17:398–406
Yang ST, Sheng GD, Guo ZQ, Tan XL, Xu JZ, Wang XK (2012) Investigation of radionuclide 63Ni(II) sequestration mechanisms on mordenite by batch and EXAFS spectroscopy study. Science China Chem 55:632–642
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Shao DD, Li JX, Wang XK (2014) Poly(amidoxime)-reduced graphene oxide composites as adsorbents for the enrichment of uranium from seawater. Science China Chem 57:1449–1458
Schlegel ML, Pointeau I, Coreau N, Reiller P (2004) Mechanism of europium retention by calcium silicate hydrates: an EXAFS study. Environ Sci Technol 38:4423–4431
Stumpf T, Curtis H, Walther C, Dardenne K, Ufer K, Fanghanel T (2007) Incorporation of Eu(III) into hydrotalcite: a TRLFS and EXAFS study. Environ Sci Technol 41:3186–3191
Sun Y, Yang S, Chen Y, Ding C, Cheng W, Wang X (2015) Adsorption and desorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study. Environ Sci Technol 49:4255–4262
Gustafsson JP (2010) Visual MINTEQ ver. 3.0. http://www2.lwr.kth.se/English/OurSoftware/vminteq/index.htm
Sheng GD, Shao DD, Fan QH, Xu D, Chen YX, Wang XK (2009) Effect of pH and ionic strength on sorption of Eu(III) to MX-80 bentonite: batch and XAFS study. Radiochim Acta 97:621–630
Hu J, Chen C, Sheng G, Li J, Chen Y, Wang X (2010) Adsorption of Sr(II) and Eu(III) on Na-rectorite: effect of pH, ionic strength, concentration and modelling. Radiochim Acta 98:421–429
Li J, Chen C, Zhang S, Wang X (2014) Comparison of adsorption–desorption hysteresis of metal cation and anion ions from carbon nanotubes. Environ Sci 1:488–495
Li J, Chen C, Zhang S, Ren X, Tan X, Wang X (2014) Critical evaluation of adsorption–desorption hysteresis of heavy metal ions from carbon nanotubes: influence of wall number and surface functionalization. Chem Asian J 9:1144–1151
Song WC, Shao DD, Lu SS, Wang XK (2014) Simultaneous removal of uranium and humic acid by cyclodextrin modified graphene oxide nanosheets. Sci China Chem 57:1291–1299
Geckeis H, Rabung Th, Ngomanh T, Kim JI, Beck HP (2002) Humic colloid-borne natural polyvalent metal ions: dissociation experiment. Environ Sci Technol 36:2946–2952
Bradbury MH, Baeyens B (2002) Sorption of Eu on Na- and Ca-montmorillonites: experimental investigations and modelling with cation exchange and surface complexation. Geochim Cosmochim Acta 66:2325–2334
Acknowledgments
Financial support from National Natural Science Foundation of China (21375148) and the Fundamental Research Funds for the Central Universities are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., He, J., Chen, L. et al. Sorption and desorption properties of Eu(III) on attapulgite. J Radioanal Nucl Chem 307, 1093–1104 (2016). https://doi.org/10.1007/s10967-015-4252-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4252-9