Abstract
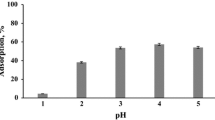
This work reports the adsorption of strontium(II) from aqueous solutions onto calcium alginate (CA) and chitosan (CH) materials. The adsorption process was studied in batch experiments as a function of the pH of the solution, contact time, and temperature. Freundlich isotherm was found to describe the sorption process comparatively well as the Langmuir model. Laboratory obtained spherical beads of CA seems to be a better sorbent than commercially available materials. A contact time of about 4 h and neutral pH of the initial aqueous solution seem to be optimal conditions for Sr-85 to be removed from contaminated solutions using alginate beads.







Similar content being viewed by others
Notes
Appropriate theoretical calculations were carried out by using the SPARTAN 2008 program [22] running on the PC. Geometry optimization and calculation of the atomic charges in the d-glucosamine and N-acetyl-d-glucosamine were performed at the Hartree–Fock level within the model UHF/3-21G(*). In particular, Natural Atomic Charges of the amine group decrease from −0.9246 and −0.7600 to −0.2870 and −0.3280 in the d-glucosamine and N-acetyl-d-glucosamine, respectively.
References
Saha GB (2004) Fundamentals of nuclear pharmacy. 5th ed. Springer, New York, pp 60, 130, 346
Nightengale B, Brune M, Blizzard SP, Ashley-Johnson M, Slan S (1995) Strontium chloride Sr 89 for treating pain from metastatic bone disease. Am J Health Syst Pharm 52:2189–2195
Robinson RG, Preston DF, Schiefelbein M, Baxter KG (1995) Strontium-89 therapy for the palliation of pain due to osseous metastases. J Am Med Assoc 274:420–424
Amano H, Yanase N (1990) Measurement of 90Sr in environmental samples by cation-exchange and liquid scintillation counting. Talanta 37:585–590
Stella R, Valentini MTG, Maggi L (1993) Determination of 90Sr and milk by using two inorganic exchangers. Appl Radiat Isot 44:1093–1096
Wüthrich M, Mauch H (1975) Wasseraufbereitung durch umgekehrte osmose. [Water Treatment by Reversed Osmosis.]. Tech Mitt PTT, 53:252–263
Benzi P, Operti L, Volpe P (1988) On the reliability of a rapid method for the determination of 90Sr in natural samples. J Radioanal Nucl Chem 126:245–256
Bojanowski R, Knapinska-Skiba D (1990) Determination of low-level 90Sr in environmental materials: a novel approach to the classical method. J Radioanal Nucl Chem 138:207–218
Blackbum R, Al-Masri MS (1993) Radioassay of strontium-90 in the presence of calcium-45 and radiocaesium (134Cs and 137Cs). Appl Radiat Isot 44:683–686
Rudd EJ, Walton CW (eds) (2000) Environmental aspects of electrochemical technology: radiological decontamination. The Electrochemical Society, Pennington
Naja GM, Volesky B (2009) Treatment of metal-bearing effluents: removal and recovery. Taylor & Francis and CRC Press, Boca Raton
Mimura H, Ohta H, Akiba K, Onodera Y (2001) Uptake behaviour of americium on alginic acid and alginate polymer gels. J Radioanal Nucl Chem 247:33–38
Dabbagh R, Ghafourian H, Baghvand A, Nabi GR, Ahmadi Faghih MA, Riahi H (2007) Bioaccumulation and biosorption of stable strontium and 90Sr by Oscillatoria homogenea cyanobacterium. J Radioanal Nucl Chem 272:53–59
Barot NS, Bagla HK (2012) Biosorption of radiotoxic 90Sr by green adsorbent: dry cow dung pow-der. J Radioanal Nucl Chem 294:81–86
Gok C, Gerstmann U, Aytas S (2013) Biosorption of radiostrontium by alginate beads. Application of isotherm models and thermodynamic studies. J Radioanal Nucl Chem 295:777–788
Imessaoudene D, Bouzidi A, Hanini S (2013) Biosorption of strontium from aqueous solutions onto spent coffee grounds. J Radioanal Nucl Chem 298:893–902
Freiser H, Nancollas GA (1987) Compendium of analytical nomenclature, 2nd edn, chap 3. Definitive rules 1987 IUPAC. Blackwell Scientific Publications, London
Chemical equilibrium diagrams: https://sites.google.com/site/chemdiagr/2013
Fuks L, Fidelis I (1987) Thermodynamic studies of complex formation of actinyl ions extracted with TBP from hydrochloric and nitric acids. J Radioanal Nucl Chem Lett 118:361–368
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions ontoChlorella vulgaris. Process Biochem 38:89–99
Townley RR, Whitney WB, Felsing WA (1937) The solubilities of barium and strontium car-bonates in aqueous solutions of some alkali chlorides. J Am Chem Soc 59:631–633
’08, Wavefunction Inc., Irvine CA, USA, 2006-2009; ISBN978-1-890661-38-4
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Cheong M, Zhitomirsky I (2008) Electrodeposition of alginic acid and composite films. Colloids Surf A: Physicochem Eng Asp 328:73–78
Soares JP, Santos JE, Chierice GO, Cavalheiro ETG (2004) Thermal behavior of alginic acid and its sodium salt. Ecl Quim 29:57–63
Acknowledgments
This research has been financed from the National Centre for Research and Development (Poland) through the Strategic Program "Technologies Supporting Development of Safe Nuclear Power Engineering", task 4 "Development of spent nuclear fuel and radioactive waste management techniques and technologies".
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuks, L., Oszczak, A., Gniazdowska, E. et al. Calcium alginate and chitosan as potential sorbents for strontium radionuclide. J Radioanal Nucl Chem 304, 15–20 (2015). https://doi.org/10.1007/s10967-014-3698-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3698-5