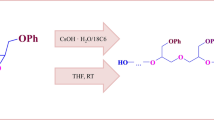
Abstract
Several new polyether-diols were prepared by ring-opening polymerization of monosubstituted oxiranes in the presence of anhydrous potassium hydroxide. 1,2-Butylene oxide (BO), styrene oxide (SO), isopropyl glycidyl ether (IPGE), allyl glycidyl ether (AGE), phenyl glycidyl ether (BGE), p-methoxyphenyl glycidyl ether (MPGE) and benzyl glycidyl ether (BGE) were chosen as monomers. Macrocyclic ligands complexing metal cations, i.e. coronand 18C6 or cryptand C222 were used as activators in these systems. All polymerizations were carried out in tetrahydrofuran solution at room temperature. Molar mass (Mn) and dispersity (Mw/Mn) of the polymers obtained with KOH depends on the kind of monomer, initial concentration of the initiator and the presence and kind of ligand or water. For example, PBO-diols prepared without ligand are bimodal and for the main fraction Mn > Mcalc. However, after addition of 18C6 polymers are unimodal and unexpectedly have much higher Mn = 13,700–15,800 and very low dispersity (Mw/Mn = 1.04–1.08). Mn of PBO-diols decrease with increase of [KOH]o and do not change at [BO]o = 2.0–9.0 mol/dm3. Addition of C222 results in Mn decrease of PBO-diols. Similar effects were observed in the polymerization of PAGE-diols and PPGE-diols. In the polymerization of SO, PGE, MPGE and BGE initiator efficiency (f) is high and Mn < Mcalc. Polymodality of some polymers obtained was discussed in term of the formation of various species propagating with different rate constants.

Polyether-diols synthesized using anhydrous hydroxide as initiator.
Similar content being viewed by others
Introduction
Polyethers, especially poly(propylene oxide)s belong to important class of polymers due to their thermal and chemical stability as well physical and mechanical properties resulting in wide applications such as impact modifiers, surfactants, de-emulsifiers, dispersant agents, fuel additives, wetting agents, lubricants, rheological modifiers, biomedical materials and adhesives [1,2,3,4,5,6,7]. Polyether–diols, i.e. homo- and copolymers prepared from ethylene oxide (EO), propylene oxide (PO) or 1,2-butylene oxide (BO) are very useful components in the fabrication of polyurethane elastomers or foams [8].
Propylene oxide have been polymerized by many procedures involving ring-opening polymerization (ROP) in bulk [9,10,11]. The most popular anionic initiators used are alkali metal alkoxides or hydroxides. It was observed, that KOH, RbOH and CsOH are effective initiators, while LiOH and NaOH are ineffective ones, probably due to their very low solubility in liquid monomer [12].
It was established by Steiner et al. [13], that PO polymerization initiated by solid anhydrous KOH is not surface-catalyzed. On the other hand, Pluciński et al. [14] assumed later, that this polymerization may be initiated both homogeneously and on the surface of KOH. The polymerization proceeds readily in bulk at 30 °C and the product obtained had high unsaturation and molar mass (Mn) about 5000 regardless of the monomer-to-catalyst ratio [13]. During the polymerization in tetrahydrofuran (THF) solution great amount of macromolecules with unsaturated starting groups also appears, resulting from deprotonation of the monomer mainly by initiator [15]. This side reaction is undesired, because it causes formation of monool fraction and decrease Mn of the polymers obtained. It was stated, that addition of water and complexing agent 18-crown-6 (18C6) strongly reduced PPOs unsaturation [15]. PPO-diols with very low unsaturation are synthesized in industry by bulk polymerization of PO in the presence of KOH/1,2-propylene glycol system at 105–125 °C and 0.3–0.5 MPa [8, 16]. Similar kind of initiator, i.e. monopotassium salt of dipropylene glycol activated by 18C6 was recently used for preparation of polystyrene-diols in THF solution at room temperature [17]. PPO was also used as a component of bifunctional polymeric activators (PACs) used for ROP of the εCL monomer, which influenced on the phase behavior of the synthesized copolymers and hence their the mechanical properties and the crystallinity degree [18].
Polyether-diols derived from some glycidyl ethers were obtained by Stolarzewicz [19, 20]. The author investigated the influence of dimethylsulfoxide and its mixtures with anisole on the KOH - initiated polymerization of o-chlorophenyl glycidyl ether [19]. It was concluded, that both solid and dissolved KOH are indispensable for initiating the polymerization with initial reaction step taking place on the surface of the solid KOH. It can be assumed, that the sorption of the monomer takes place on the surface of the undissolved KOH. The adsorbed monomer then reacts with a dissolved KOH molecule, which leads to ring opening and initiation of the polymerization. Moreover, in the series of chlorophenyl glycidyl ethers polymerization, involving monomers with different number of chlorine atoms, in the presence of KOH the substituent effect attention was paid to decrease of the basicity of the reaction mixture during the polymerization [20]. This phenomenon was assigned to lability of chlorine atoms bonded to the aromatic ring and on additional consumption of the initiator in the reaction with Cl-substituted monomer leading to OH-substituted ones.
Other examples of polyether-diols involve copolyether-diols synthesized from various oxiranes, i.e. block copolymers PO-EO with terminal PEO blocks, block copolymers PO-EO with internal PEO blocks and random copolymers PO-EO [8]. They are used especially for polyurethane elastomers, coatings, adhesives and sealants [21, 22]. The most extensively studied triblock copolyether-diol is PEO/PPO/PEO, polymeric nonionic surfactant, which is commercially available as Pluronics (BASF) or Synperonics (ICI) [23]. In this polymers PPO builds the hydrophobic block, whereas PEO two hydrophilic blocks. Then, a number of ABA and BAB triblock copolymers of ethoxyethyl glycidyl ether and PO were prepared by sequential anionic polymerization initiated with dicesium salt of 1,2-propylene glycol [24]. It was stated, that these copolymers can form nanosized aggregates and exhibit different temperature behavior, depending on the copolymer architecture. However, data concerning synthesis and application of other polyether-diols are rather scarce [8].
The aim of the present work was characterization of several new polyether-diols prepared in anionic ring-opening polymerization of other monosubstititued oxiranes initiated with anhydrous KOH, which till now was applied mainly for polymerization of propylene oxide [13,14,15]. Some of them may be interesting as substrates for the synthesis of new thermoplastic polyurethanes. 1,2-Butylene oxide (BO), styrene oxide (SO) and some glycidyl ethers, such as isopropyl glycidyl ether (IPGE), allyl glycidyl ether (AGE), phenyl glycidyl ether (PGE), p-methoxyphenyl glycidyl ether (MPGE) and benzyl glycidyl ether were chosen as monomers. All polymerizations were carried out in THF solution and mild conditions, i.e. room temperature and normal pressure. The influence of initial concentration of the monomer, initiator, the presence of water and kind of macrocyclic ligand complexing counterion (L), i.e. coronand 18C6 or cryptand C222 on molar mass, dispersity and modality of the prepared polyether-diols were studied and discussed. 13C NMR, MALDI-TOF and SEC techniques were used for analysis of the polymers. We decided to develop the studies taking into account that data concerning polymerization of mentioned oxiranes with anhydrous KOH are scare. The present results concerning the influence of mentioned parameters on molar masses and modality of synthesized polyether-diols are novel and develop our knowledge about anionic polymerization of oxiranes.
Experimental
Materials
Monomers, i.e. 1,2-butylene oxide, styrene oxide, isopropyl glycidyl ether, allyl glycidyl ether, phenyl glycidyl ether, p-methoxyphenyl glycidyl ether and benzyl glycidyl ether (all from Aldrich) were dried over CaH2 and distilled at 336 K (63 °C), 467 K (194 °C), 414 K (131 °C), 427 K (154 °C), 518 K (245 °C), 350 K (77 °C)/10 Torr and 344 K (71 °C)/11 Torr, respectively. Anhydrous tetrahydrofuran (THF) (Acros Organics) was kept over CaH2 and distilled at 339 K (66 °C). Potassium hydride (KH) was purified according to the procedure described by Brown [25]. A 35 wt-% dispersion of KH in mineral oil (Aldrich) was mixed with n-pentane in a dry argon atmosphere and then decanted. This was repeated three times followed by a three-fold washing with dry THF. Finally, THF was evaporated in vacuum. Coronand 18C6 (1,4,7,10,13,16-hexaoxacyclooctadecane) (Merck) and cryptand C222 (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8,8,8]hexacosane) (Merck) were used without purification.
Synthesis
All syntheses were performed at 20 °C in a 50 cm3 reactor equipped with a magnetic stirrer and a Teflon valve enabling substrates delivery and sampling under argon atmosphere. Potassium hydroxide was obtained in the reaction of potassium hydride with distilled water dissolved in tetrahydrofuran. In the first series of polymerizations of 1,2-butylene oxide the initial concentration of the monomer was equal to 2.0 mol/dm3 and the initial amount of potassium hydroxide was 0.28, 0.56 or 0.84 (0.05, 0.10 and 0.15 mol/dm3, respectively). For example, potassium hydride (0.08 g, 2.0 mmol) and tetrahydrofuran (16.5 cm3) was introduced into the reactor and then water (0.036 g, 2.0 mmol) was added by use of microsyringe. The reaction mixture was stirred during 2 h until all hydrogen (44.7 cm3) was evolved. It resulted in a fine dispersion of pure anhydrous potassium hydroxide in the ether medium. That system was used as the initiator when 1,2-butylene oxide (3.5 cm3, 0.42 g, 40 mmol) was introduced into the reactor. The reaction mixture was then stirred during several days. After complete conversion of the monomer the reaction mixture was neutralized with HCl/H2O system (0.1 mol/dm3, 50 cm3) and transferred to the separator containing chloroform (70 cm3). After shaking during 5 min, two layers were formed, i.e. interferior polyether layer and superior layer containing water and the potassium salt. These layers were separated and the superior layer was removed. After three washings with distilled water, polyether was obtained by evaporating of chloroform and water in vacuum. In the next series of polymerizations KOH was obtained in the reaction of KH with H2O in the presence of 18C6 (0.53 g, 2.0 mmol) or KH/C222 (0.75 g, 2.0 mmol). Initiator KOH/18C6/H2O (1/1/0.5) was prepared in the reaction of KH/18C6 with 0.054 g (3.0 mmol) H2O. Similar procedure was applied for polymerization of other oxiranes. The concentration of monomer during polymerization was monitored by the 1,4-dioxane method [26]. The final conversions were ~99%. The yields of the reactions were 97–99%. Almost all investigated processes were heterogeneous and initiators were present at the end of the polymerization.
Measurements
100 MHz 13C NMR spectra were recorded in CDCl3 at 25 °C on a Bruker Avance 400 pulsed spectrometer equipped with 5 mm broad-band probe and applying Waltz16 decoupling sequence. Chemical shifts were referenced to tetramethylsilane serving as an internal standard. To obtain a good spectrum of the polymer main chain exhibiting its microstructural details about 3000 scans were satisfactory but in order to observe the signals of the polymer chain ends more than 10,000 scans were necessary. Molar masses and dispersities of polymers were obtained by means of size exclusion chromatography (SEC) on a Shimadzu Prominance UFLC instrument at 40 °C on a Shodex 300 mm × 8 mm OHpac column using tetrahydrofuran as a solvent. Polystyrenes were used as calibration standards. MALDI-TOF spectra were recorded on a Shimadzu AXIMA Performance instrument. Dithranol was used as a matrix.
Results and discussion
The results presented below concern the most important and interesting phenomena observed in the polymerization of chosen oxiranes initiated with anhydrous KOH. Precisely four kinds of initiators were applied, namely pure KOH in the form of dispersion, KOH activated by coronand 18C6, partially hydrated KOH activated by 18C6 and KOH activated by cryptand C222.
Polymerisation of 1,2-butylene oxide
It was interesting whether initiation is mediated by KOH solubilized in the reaction mixture or on crystal surface. Table 1 presents SEC results concerning heterogeneous polymerization of 1,2-butylene oxide (BO) carried out at various initial amount of KOH.
It was assumed, that KOH reacts in the system by two ways, i.e. ring opening (RO) of the monomer and deprotonation of OH group. RO results in hydroxyalkoxide formation, while deprotonation of OH group leads to OK ones and further to H2O and dialkoxide, which mediates chain propagation in two directions. It was also postulated, that interaction of KOH with H2O leads to the formation of hydrate KOH·H2O. It was stated in additional experiment, that hydrate KOH·H2O (1/1) is insoluble in the reaction mixture and does not initiate BO polymerization, similarly as it was observed previously for PO [15]. The course of the polymerization was presented on Fig. 1.
Formation of PBO-diol macromolecules was confirmed by MALDI-TOF analysis of polymers. Figure 2 shows spectrum of exemplary polymer (3).
Spectrum at m/z 900 to 6000 reveals signals, which belong exclusively to macromolecules containing central oxygen atom and two terminal OH groups. These macromolecules form adducts with sodium ions. For example, signals at m/z 1554.7; 2130.2 and 2778.1 represents macromolecules, which contain 21, 29 and 38 mers of BO, respectively (Mcalc = 1555.3; 2132.2 and 2781.2, respectively).
13C NMR spectrum of this polymer reveals carbon signals of CH2 (at 71.5–72.5 ppm) and CH (at 80.6–81.0 ppm) groups in polymer chain as well as CH3 (at 9.9 ppm) and CH2 (at 24.9 ppm) groups present in substituents. Weak signal of CH(CH2CH3)OH terminal groups was detected at 74.1 ppm. However, none signals at 90–150 ppm were observed, which indicated the lack of unsaturation, resulting from deprotonation of monomer with initiator and chain transfer reaction.
It is worth noting, that prepared PBO-diols are bimodal and have low dispersities (Table 1, Fig. 3).
Analyzing polymerization of some oxacyclic compounds, Penczek et al. [27] confirmed the possibility of occurrence of bimodal molar mass distribution. They stated that in the polymerization process bimodality exclusively appeared, when there were two species propagating with different rate constants and that these species did not exchange fast enough. Therefore, we propose, that in the polymerization of BO initiated with KOH two kinds of alkoxide centers with different reactivity are formed (Fig. 4) and they are responsible for the observed bimodality.
However, molar masses of the main fractions of PBOs (1–3) are higher than calculated ones. It means, that part of initiator remains inactive and does not initiate polymerization. It involves insoluble KOH present in crystal lattice as well as hydrate KOH·H2O. Moreover, Mn of both fractions depends on initial amount of KOH in the system, i.e. decrease with [KOH]o. This result differs strongly from that observed by Steiner et al. [13] in the polymerization of PO carried out in similar conditions. In that case Mn of PPOs was independent on the amount of KOH used for initiation. Even at a catalyst-to-propylene oxide mole ratio of one there was no significant change in reaction rate nor in product characteristic. The author assumed, that the polymerization proceeds in this case as a process initiated by KOH dissolved partly in the monomer. However, Pluciński [14] proposed, that PO polymerization may be initiated both homogeneously and on the surface of KOH. Similar effect was reported by Stolarzewicz [19] for o-chlorophenyl glycidyl ether. The results obtained by us for BO polymerization lead to the same conclusions. Concentration of KOH solubilised in the reaction mixture is rather low and constant in the polymerization (1)–(3). However, molar masses of the polymers decrease distinctly with increase of initial amount of KOH. It indicates, that initiation of the polymerization may occur also on the crystal surface of anhydrous KOH.
Unexpectedly, application of coronand 18C6, which forms strong complexes with K+ results in distinct shortening of polymerization time and increase of polymers molar masses (4–6) (Table 2). This effect could be explained by appearance of ligand separated highly reactive ion pairs in growing chains existing in equilibrium with contact ion pairs [28] (Fig. 5). Rate of propagation on the former is much higher than on the latter.
Molar masses of polymers (4–6) decrease with increase of KOH/18C6 amount in the system. Addition of some amount of H2O to (5) decreases markedly Mn of polymer (7) due to chain transfer reaction to water. All polymers in this series are unimodal (Fig. 6) and have low dispersity (Mw/Mn = 1.04–1.08), except polymer (7), which has relatively high dispersity (Mw/Mn = 1.31).
Interestingly, molar masses of the polymers synthesized at much higher initial concentration of the monomer, i.e. 5.0 (8) and 9.0 mol/dm3 (9) have similar values. Increase of [BO]o from 2.0 (5) to 9.0 mol/dm3 (9) results only in small increase of polymers Mn, i.e. from 14,800 to 16,300 for polymers prepared at the same [KOH]o = 0.10 mol/dm3. This effect could be explained by high increase of initiator efficiency (f) caused probably by higher polarity of the medium. It is also worth noting, that dispersity of the polymers (4, 5, 8 and 9) is very low (Mw/Mn = 1.04–1.05) and independent on initial concentration of monomer.
Polymers (10–12) synthesized in the presence of cryptand C222 have molar masses about two-times lower, than these for polymers prepared with 18C6 (Table 3). It probably results from increased solubility of initiator in the presence of C222, the strongest ligand for K+ [29]. Alkoxide anion practically does not interact with potassium cation complexed by C222 (Fig. 7). Interestingly, at lowest [KOH]o three fractions of polymer (10) are obtained (Fig. 8).
Dispersity of polymers needs some comments. In general, Mw/Mn values are very low, e.g. 1.04–1.08 for polymers obtained in the presence of 18C6. Addition of H2O distinctly increases dispersity to 1.31. Polymers prepared in the presence of C222 are polymodal or have relatively high dispersity (1.32–1.56).
Polymerisation of styrene oxide
In the course of polymerization of styrene oxide (SO) initiated with KOH activated by 18C6 the reaction mixture are dark red and homogeneous. Polymer (13) obtained at lower [SO]o is unimodal, whereas that prepared at higher [SO]o is bimodal (14) (Table 4). Polymers have relatively high dispersity and Mn of the polymers are lower than Mcalc.
It was proposed, that initiation occurs quantitatively in this case, due to high solubility of KOH in the reaction mixture. It was assumed that in this case free water does not form hydrate with KOH and remains in the solution. During the polymerization chain transfer reaction to water occurs, which lowering Mn of the polymers obtained (Fig. 9).
MALDI-TOF spectrum of polymer (14) shown in Fig. 10 reveals one series of signals at m/z 521.6 to 4476.8, which belongs to macromolecules containing central oxygen atom and two terminal OH groups. For example, signals at m/z 762.4, 2322.1 and 3400.6 represents macromolecules which contain 6, 19 and 28 mers of the monomer, respectively (Mcalc = 761.9, 2323.8 and 3405.2, respectively). These macromolecules form adducts with sodium ions.
Styrene oxide is monosubstituted oxirane, which possesses acidic hydrogen atom in the molecule. It is responsible for deprotonation of the monomer by initiator and alkoxide centre of growing chain (chain transfer reaction), which occurs as side reactions in anionic polymerization of theses oxiranes. Recently, we reported [17], that PSOs prepared in polymerization initiated with potassium alkoxides indicates some unsaturation, resulting from deprotonation of methine group (and not methylene ones) present in SO molecule. Signals of carbons of unsaturated starting groups CH2 = C(Ph)O– were observed at 133.02 and 159.66 ppm in 13C NMR spectrum. However, these signals were not detected in the spectra of PSO-diols obtained in this work. Evidently, chain transfer to water is much faster than to the monomer, which results in lack of polymers unsaturation.
Polymerisation of glycidyl ethers
Formation of bimodal polymers is also observed in the polymerization of allyl glycidyl ether (AGE) initiated in the presence of KOH without ligand (15) (Table 5).
Similarly to the polymerization of BO, application of 18C6 results in the formation of unimodal polymer (16) with markedly higher Mn = 11,000 and very low dispersity (Mw/Mn = 1.03). Molar mass and dispersity of polymer (17) prepared at much higher [mon]o is near the same. It probably results from distinct increase of initiator efficiency, probably due to higher polarity of the medium. Addition of small amount of H2O results in polymer (18) polymodality and decrease of Mn. Similar effect is observed in the presence of C222 (19).
13C NMR analysis of polymer (15) prepared without ligand indicated signals of unsaturated allyl groups present in substituents in polymer chain at 116.9 and 134.9 ppm (Fig. 11a). However, in the spectrum of polymer (16) obtained in the presence of 18C6 signals of cis-propenyl groups are also observed (Fig. 11b). They consist 12% of total unsaturation. Stronger signals of cis-propenyl groups were shown in the spectrum of polymer (19) synthesized in the presence of C222 (Fig. 11c). They consist 28% of total unsaturation. Isomerization of side allyl groups to cis-propenyloxy ones was observed previously in anionic polymerization of propylene oxide [8, 13] and never in anionic polymerization of AGE
It was proposed, that due to steric hindrance isomerization is mediated with KOH activated by ligand and not with alkoxide anion of growing chain, as it was reported previously in the polymerization of propylene oxide (PO) [13, 15]. Fig. 12 presents possible course of the reaction.
Molar mass of PIPGE-diol (20) is higher than Mcalc, which means that initiator efficiency is high in this system. However, polymerization of BGE (21) is homogeneous, similarly to that of SO (13), which results in high decrease of Mn due to chain transfer to water.
In general, in the polymerization of PGE similar effects of ligands on molar masses are observed as in the polymerization of other oxiranes. It is also worth noting, that in the polymerization of MPGE the phenomenon assigned to lability of CH3O group bonded to the aromatic ring under influence of KOH was not observed. 13C NMR spectra of the polymers (27) and (28) indicated exclusively signal of CH3O group bonded to the aromatic ring (at 55.7 ppm); signal of CH3OCH2CH(R)O was absent. High initiator efficiency were also calculated for polymerization of phenyl glycidyl ether and p-methoxyphenyl glycidyl ether (Table 6).
Polymodality of polyether-diols observed in some studied systems need comments. Such effect depends on the kind of monomer, ligand and water. For example, bimodal PAGE-diol was obtained in the polymerization initiated KOH without ligand, but three fractions of PBO-diol with KOH activated C222 was formed. Presumably, various ionic species with different reactivities taking part in chain propagation, i.e. contact ion pairs, solvated ion pairs, ligand separated ion pairs and other ionic aggregates are responsible for this phenomenon [27, 28]. On the other hand, by using for initiation monopotassium salt of dipropylene glycol in THF solution homogeneous polymerization of the same oxiranes were carried out, comparitavely. In these system exclusively unimodal polyether-diols were obtained. Their molar masses determined by SEC are similar to theoretical ones (Mn ≈ Mcalc). Assuming, some additional experiments applying of the more polar solvents, kinetic study and model reactions may help to better understand the nature and the action of the studied monomers and initiating systems.
Conclusions
Anhydrous potassium hydroxide (KOH) was applied for ring-opening polymerization of monosubstituted oxiranes in THF at room temperature. Several systems were activated by macrocyclic ligands complexing metal cations. Main characteristic features of these processes are:
-
Molar mass (Mn), dispersity (Mw/Mn) and modality of the polymers obtained in the presence of KOH depends strongly on the kind of the monomer, initial concentration of the initiator and the presence and kind of ligand and water;
-
PBO-diols prepared without ligand are bimodal and Mn > Mcalc; after addition of 18C6 the polymers are unimodal and unexpectedly they have markedly higher Mn (13700–15,800) and very low dispersity (Mw/Mn = 1.04–1.08);
-
Mn of PBO-diols decreases with increasing of [KOH]o and only slightly increase at [BO]o = 2.0–9.0 mol/dm3, probably due to increase of [KOH]o activated 18C6 in much more polar reaction mixture; addition of C222 causes lowering of Mn for PBO-diols; similar effects were observed for PAGE-diols and PPGE-diols;
-
PSO-diols prepared at low [SO]o is unimodal, whereas at higher [SO]o is bimodal and the systems are homogeneous;
-
In the polymerization of SO, PGE, MPGE and BGE initiator efficiency is high, resulting in Mn < Mcalc and chain transfer to water formed during initiation;
-
In all obtained polymers unsaturation was not observed; it means, that chain transfer to monomer was absent;
-
Polymodality of some polymers can be explained by the formation of various ionic species propagating with different rate constants;
-
Some of prepared polyether-diols could be useful for synthesis of new polyurethane elastomers due to their relatively high Mn and low dispersity.
References
Gosa KI, Uricanu V (2002) Emulsions stabilized with PEO–PPO–PEO block copolymers and silica. Colloids Surf A Physicochem Eng Asp 197:257–269
Mathur AM, Drescher B, Scranton AB, Klier J (1998) Polymeric emulsifiers based on reversible formation of hydrophobic units. Nature 392:367–370
Herzberger J, Niederer K, Pohlit H, Seiwert J, Worm M, Wurm FR, Frey H (2016) Polymerization of ethylene oxide, propylene oxide, and other alkylene oxides: synthesis, novel polymer architectures, and bioconjugation. Chem Rev 116:2170–2243. https://doi.org/10.1021/acs.chemrev.5b00441
Zhang ZQ, Xu GY, Wang F, Dong SL, Li Y (2004) Characterization and demulsification of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) copolymers. J Colloid Interface Sci 277:464–470
Jeong B, Bae YH, Lee DS, Kim SW (1997) Biodegradable block copolymers as injectable drug-delivery systems. Nature 388:860–862
Izukawa T, Kunihiro T, Nishikawa A (Eds.) M.C.I. US Patent 5, 916, 994, 1999
Cendejas G, Flores-Sandoval CA, Huitrón N, Herrera R, Zamudio-Rivera LS, Beltrán HI, Vázquez F (2008) Theoretical and experimental studies of the initiator influence on the anionic ring opening polymerization of propylene oxide. J Mol Struct 879:40–52
Ionescu M (2005) Chemistry and Technology of Polyols for Polyurethanes, Rapra Technology Limited, Shawbury, Shrewsbury, Shropshire and references therein
Becker H, Wagner G (1984) Zur Übertragungsreaktion bei der anionichen Polymerisation von Oxiranen VI. Zum Einfluß von Kronenetherzusätzen auf die Polymerisation von Propylenoxid. Acta Polym 35:28–32
Yu GE, Heatley F, Booth C, Bleas TG (1994) Anionic polymerization of propylene oxide: isomerization of allyl ether to propenyl ether end groups. J Polym Sci A Polym Chem 32:1131–1135
Ding J, Price C, Booth C (1991) Use of crown ether in the anionic polymerization of propylene oxide-1. Rate of polymerization. Eur Polym J 27:891–894
Pierre LES, Price CC (1956) The room temperature polymerization of propylene oxide. J Am Chem Soc 78(14):3432–3436. https://doi.org/10.1021/ja01595a047
Steiner EC, Pelletier RR, Trucks RO (1964) A study of the polymerization of propylene oxide catalyzed by anhydrous potassium hydroxide. J Am Chem Soc 86:4678–4686
Pluciński J, Matyschok H, Janik R, Prystasz H (1981) Polymerisation des Propylenoxids in Anwesenheit von fastem Kaliumhydroxid. Angew Macromol Chem 97:35–50
Grobelny Z, Matlengiewicz M, Jurek J, Michalak M, Kwapulińska D, Swinarew A, Schab-Balcerzak E (2013) The influence of macrocyclic ligands and water on propylene oxide polymerization initiated with anhydrous potassium hydroxide in tetrahydrofuran. Eur Polym J 49:3277–3288
Wegner G, Brandt M, Duda L, Hofmann J, Kleszczewski B, Koch D et al (2001) Trends in industrial catalysisin the polyurethane industry. Appl Catal A General 221:303–305
Grobelny Z, Matlengiewicz M, Jurek-Suliga J, Golba S, Skrzeczyna K, Kwapulińska D (2017) Ring opening polymerization of styrene oxide initiated with potassium alkoxides and hydroxyalkoxides activated by 18-crown-6: determination of mechanism and preparation of new polyether-polyols. Polym Bull 74:4763–4780
Zhilkova K, Mateva R, Kyulavska M (2017) Copolymers of ε-caprolactam and polypropylene oxide via anionic polymerization: synthesis and properties. J Polym Res 24:162–172. https://doi.org/10.1007/s10965-017-1324-2
Stolarzewicz A (1983) Influence of the structure of chlorophenyl 2,3-epoxypropyl ethers on their reactivity in ionic polymerization. Macromol Chem 184:1577–1584
Stolarzewicz A (1983) Application of correlation equations for determining the solvent effect in the heterogeneous polymerization of o-chlorophenyl glycidyl ether initiated by potassium hydroxide. Acta Polym 34:210–212
Lambda NMK, Woodhouse KA, Cooper CL (1998) Polyurethanes in biomedical applications. CRC Press, New York
Meier-Westhues U (2007) Polyurethanes-coatings, adhesives and Sealants, Vincentz Network Gmbh, Hannover
Malmsten M, Linse P, Zhang KW (1993) Phase behavior of aqueous poly(ethylene oxide)/poly(propylene oxide) solutions. Macromolecules 26:2905–2910
Dimitrov P, Rangelov S, Dworak A, Tsvetanov CB (2004) Synthesis and associating properties of poly(ethoxyethyl glycidyl ether)/poly(propylene oxide) triblock copolymers. Macromolecules 37:1000–1008
Brown CA (1974) Saline hydrides and superbases in organic reactions. VII. Potassium hydride, highly active new hydride reagent. Reactivity, applications, and techniques in organic and organometallic reactions. J Org Chem 39:3913–3918
Siggia S (1963) Quantitative organic analysis via functional groups. Wiley, New York, p 241
Penczek S, Cypryk M, Duda A, Kubisa P, Słomkowski S (2007) Living ring-opening polymerizations of heterocyclic monomers. Prog Polym Sci 32:247–282
Szwarc M (ed) (1974) Ion and ion pairs in organic reactions. Wiley, New York
Izatt RM, Bradshaw JS, Nielsen SA, Lamb JD, Christensen JJ (1985) Thermodynamic and kinetic data for cation-macrocycle interaction. Chem Rev 85:271–339
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Grobelny, Z., Golba, S. & Jurek-Suliga, J. Characterization of new polyether-diols with different molar masses and modality prepared by ring opening polymerization of oxiranes initiated with anhydrous potassium hydroxide. J Polym Res 26, 75 (2019). https://doi.org/10.1007/s10965-019-1727-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1727-3