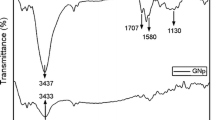
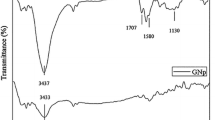
Abstract
In present study, the effect of graphene (GR) on crystallization and thermal degradation kinetic behavior of poly(lactic acid) (PLA) was investigated. The non isothermal cold crystallization kinetic study for PLA-GR nanocomposites was carried out using differential scanning calorimetry at different heating rates of 2.5, 5, 7.5 and 10 °C/min. The obtained kinetic data were analyzed using crystallization kinetic models such as Avrami and Tobin methods. The decreasing trend obtained in the Avrami as well as Tobin exponent (n and n T, respectively) with respect to neat PLA revealed the nucleating effect of graphene. Thermal degradation behavior of both PLA and PLA-GR nanocomposites was also analyzed by Kissinger method. The increasing trend in the activation energy with respect to GR loading was observed as compared to neat PLA. This is an indication of improvement in the thermal stability of PLA with an increase in the GR loading. Polarized optical microscopy (POM) was used to observe the growth of spherulites in the PLA and PLA-GR nanocomposites. With respect to addition of GR in the PLA matrix, reduction in the nucleation induction time and an increment in the number of nucleation sites were reflected in the POM analysis.













Similar content being viewed by others
References
Goffin AL, Raquez JM, Duquesne E, Siqueria G, Habibi Y, Dufresne A, Dubois P (2011) From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromolecules 12:2456–2465
Ray SS, Bousmina M (2005) Biodegradable polymers and their layered silicate nanocomposites: in greening the 21st century materials world. Prog Mater Sci 50:962–1079
Liu H, Song W, Chen F, Guo L, Zhang J (2011) Interaction of microstructure and interfacial adhesion on impact performance of polylactide (PLA) ternary blend. Macromolecules 44:1513–1522
Hoglund A, Hakkarainen M, Albertson AC (2010) Migration and hydrolysis of hydrophobic polylactide plasticizer. Biomacromolecules 11:277–283
Ljungberg N, Wesslen B, Preparation and properties of plasticized poly(lactic acid) films. Biomacromolecules 6: 1789–1796.
Ray SS (2012) Polylactide-based bionanocomposites: a promising class of hybrid materials. Acc Chem Res 45:1710–1720
Wu D, Cheng Y, Feng S, Yao Z, Zhang M (2013) Crystallization behavior of polylactide/graphene composites. Ind Eng Chem Res 52:6731–6739
Mubarak Y, Harkin-Jones EMA, Martin PJ, Ahmad M (2001) Modeling of non-isothermal crystallization kinetics of isotactic polypropylene. Polymer 42:3171–3182
Liu Y, Wang L, He Y, Fan Z, Lia S (2010) Non-isothermal crystallization kinetics of poly(L-lactide). Polym Int 59:1616–1621
Tudorachi N, Lipsa R, Mustata FR (2012) Thermal degradation of carboxymethyl starch–g-poly(lactic acid) copolymer by TG–FTIR–MS analysis. Ind Eng Chem Res 51:15537–15545
Khawan A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110:17315–17328
Jankovic B (2008) Solid-state kinetic models: basics and mathematical fundamentals. Chem Eng J 139:128–135
Aboyade AO, Carrier M, Meyer EL, Knoetze JH, Gorgens JF (2012) Model fitting kinetic analysis and characterisation of the devolatilization of coal blends with corn and sugarcane residues. Thermochim Acta 530:95–106
Jankovic B, Adnadevic B, Jovanovic J (2007) Application of model-fitting and model-free kinetics to the study of non-isothermal dehydration of equilibrium swollen poly (acrylic acid) hydrogel: thermogravimetric analysis. Thermochim Acta 452:106–115
Peng F, Shaw MT, Olson JR, Wei M (2011) Hydroxyapatite needle-shaped particles/poly(l-lactic acid) electrospun scaffolds with perfect particle-along-nanofiber orientation and significantly enhanced mechanical properties. J Phys Chem C 115:15743–15751
Liu L, Jin TZ, Coffin DR, Hicks KB (2009) Preparation of antimicrobial M membranes: coextrusion of poly(lactic acid) and nisaplin in the presence of plasticizers. J Agric Food Chem 57:8392–8398
Katiyar V, Gerds N, Koch CB, Risbo J, Hansen HCB, Plackett D (2010) Poly l-lactide-layered double hydroxide nanocomposites via in situ polymerization of l-lactide. Polym Degrad Stab 95:2563–2573
Valapa R, Pugazhenthi G, Katiyar V (2014) Thermal degradation kinetics of sucrose palmitate reinforced poly(lactic acid) biocomposites. Int J Biol Macromol 65:275–283
Sawai D, Takahashi K, Sasashige A, Kanamoto T, Hyon SH (2003) Preparation of oriented β-form poly(l-lactic acid) by solid-state coextrusion: effect of extrusion variables. Macromolecules 36:3601–3605
Hoogsteen W, Postema AR, Pennings AJ, Brinke GT (1990) Crystal structure, conformation and morphology of solution-spun poly(L-lactide) fibers. Macromolecules 23:634–642
Bharadwaj R, Mohanty AK, Drzal LT, Pourboghrat F, Misra M (2006) Renewable resource-based green composites from recycled cellulose fiber and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic. Biomacromolecules 7:2044–2051
Ravari F, Mashak A, Nekoomanesh M, Mobedi H (2013) Non-isothermal cold crystallization behavior and kinetics of poly(l-lactide): effect of l-lactide dimer. Polym Bull 70:2569–2586
Yasuniwa M, Sakamo K, Ono Y, Kawahara W (2008) Melting behavior of poly(l-lactic acid): X-ray and DSC analyses of the melting process. Polymer 49:1943–1951
Nofar M, Zhu W, Park CB, Randall J (2011) Crystallization kinetics of linear and long-chain-branched polylactide. Ind Eng Chem Res 50:13789–13798
Fukushima K, Abbate C, Tabuani D, Gennari M, Camino G (2009) Biodegradation of poly(lactic acid) and its nanocomposites. Polym Degrad Stab 94:1646–1655
Fortunati E, Armentano I, Zhou Q, Puglia D, Terenzi A, Berglund LA, Kenny JM (2012) Microstructure and nonisothermal cold crystallization of PLA composites based on silver nanoparticles and nanocrystalline cellulose. Polym Degrad Stab 97:2027–2036
Pei A, Zhou Q, Berglund LA (2010) Microstructure and nonisothermal cold crystallization of PLA composites based on silver nanoparticles and nanocrystalline cellulose. Compos Sci Technol 70:815–821
Vasanthan N, Ly H, Ghosh S (2011) Impact of nanoclay on isothermal cold crystallization kinetics and polymorphism of poly(l-lactic acid) nanocomposites. J Phys Chem B 115:9556–9563
Wang L, Jing X, Cheng H, Hu X, Yang L, Huang Y (2012) Blends of linear and long-chain branched poly(l-lactide)s with high melt strength and fast crystallization rate. Ind Eng Chem Res 51:10088–10099
Qiu Z, Li Z (2011) Effect of orotic acid on the crystallization kinetics and morphology of biodegradable poly(l-lactide) as an efficient nucleatin. Ind Eng Chem Res 50:12299–12303
Bao RY, Yang W, Jiang WR, Liu ZY, Xie BH, Yang MB (2013) Polymorphism of racemic poly(l-lactide)/poly(d-lactide) blend: effect of melt and cold crystallization. J Phys Chem B 117:3667–3674
Hemalatha S, Maiti n (2012) Nonisothermal crystallization kinetics of PA6 and PA6/SEBS-g-MA blends. J Polym Res 19:9926–9931
Durmus A, Yalcinyuva T (2008) Effect of additives on non-isothermal crystallization kinetics and morphology of isotactic polypropylene. J Polym Res 16:489–498
Liao R, Yang B, Yu W, Zhou C (2007) Isothermal cold crystallization kinetics of polylactide/nucleating agents. J Appl Polym Sci 104:310–317
Han Q, Wang Y, Shao C, Zheng G, Li Q, Shen C (2013) Nonisothermal crystallization kinetics of biodegradable poly(lactic acid)/zinc phenylphosphonate composites. J Comp Mater 48:2737–2746
Zinet M, Reffa Z, Boutaous M, Xin S, Bourgin P (2013) Thermophysical characterization and crystallization kinetics of semi crystalline polymers. J Mod Phys 4:28–37
Andjelic S, Scogna CR (2015) Polymer crystallization rate challenges: the art of chemistry and processing. J Appl Polym Sci 132:42066–42080
El-Hadi AM, Mohan SD, Davis FI, Mitchell GR (2014) Enhancing the crystallization and orientation of electrospining poly(lactic caid) (PLLA) by combining with additives. J Polym Res 21:605–612
Jayaramudu J, Reddy SMG, Varaprasad K, Saidiku ER, Wang SS, Rajulu AV (2013) Structure and properties of poly (lactic acid)/sterculia urens uniaxial fabric biocomposite. Carbohydr Polym 94:822–828
Wang Y, Steinhoff B, Brinkmann C, Alig I (2008) In-line monitoring of the thermal degradation of poly(l-lactic acid) during melt extrusion by UV–Vis spectroscopy. Polymer 49:1257–1265
Cao Y, Feng J, Wu P (2010) Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly(lactic acid) composites. Carbon 48:3834–3839
Wang X, Xing W, Zhang P, Song L, Yang H, Hu Y (2012) Covalent functionalization of graphene with organosilane and its use as a reinforcement in epoxy composites. Compo Sci Technol 72:737–743
Yu H, Huang N, Wang C, Tang Z (2003) Modeling of poly(L-lactide) thermal degradation: theoretical prediction of molecular weight and polydispersity index. J Appl Polym Sci 88:2557–2562
Yuzay IE, Auras R, Valdez H, Selke S (2010) Effects of synthetic and natural zeolites on morphology and thermal degradation of poly(lactic acid) composites. Polym Degrad Stab 95:1769–1777
Flynn JH, Wall LA (1966) A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci 4:323–328
Chen X, Tu J, Luo Z, Hu S, Zhou Z, Guo S, Lu S (2009) Kinetics of thermo-oxidative degradation of zinc borate/microcapsulated red phosphorous with magnesium hydroxide in flame retarded polypropylene composites. J Polym Res 16:745–753
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Chrissafis K (2010) Detail kinetic analysis of the thermal decomposition of PLA with oxidized multi-walled carbon nanotubes. Thermochim Acta 511:163–167
Fan Y, Nishida H, Shirai Y, Endo T (2004) Thermal stability of poly (l-lactide): influence of end protection by acetyl group. Polym Degrad Stab 84:143–149
Yang TCK, Lin SSY, Chuang TH (2002) Kinetic analysis of the thermal oxidation of metallocene cyclic olefin copolymer (mCOC)/TiO2 composites by FTIR microscopy and thermogravimetry (TG). Polym Degrad Stab 78:525–532
Chen EC, Wu TM (2007) Isothermal crystallization kinetics and thermal behavior of poly(ɛ-caprolactone)/multi-walled carbon nanotube composites. Polym Degrad Stab 92:1009–1015
Li J, Zheng W, Lia L, Zheng Y, Lou X (2009) Thermal degradation kinetics of g-HA/PLA composite. Thermochim Acta 493:90–95
Acknowledgments
The authors sincerely thank the Department of Chemicals and Petrochemicals, Ministry of Chemicals and Fertilizers, Government of India funded Center of Excellence for Sustainable Polymers at IIT Guwahati for research facilities to perform this research work.
Ethical agreement
• The manuscript has not been submitted to more than one journal for simultaneous consideration.
• The manuscript has not been published previously.
• A single study is not split up into several parts.
• No data have been fabricated or manipulated.
• No data, text, or theories by others are presented.
• Consent to submit has been received explicitly from all co-authors before the work is submitted.
• Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Valapa, R., Hussain, S., Iyer, P.K. et al. Influence of graphene on thermal degradation and crystallization kinetics behaviour of poly(lactic acid). J Polym Res 22, 175 (2015). https://doi.org/10.1007/s10965-015-0823-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0823-2