Abstract
In this paper the preparation of surface molecularly imprinted Cu(II) catalysts with modified active centres is described. The resins were synthesized using suspension polymerization of 1-vinylimidazole, acrylonitrile, 1,1,1-tris(hydroxymethyl)propane trimethacrylate and 4-vinyloxybutylstearate in the presence of Cu(II) ions and 4-methoxybenzyl alcohol as the template. The resins obtained in the form of polymer beads posses diameters ranging from 30 μm to 300 μm and specific surface areas up to 315 m2/g depending on sonication mode. The resins, after copper removal, were modified in a two-step reaction: bromoalkylation with four alkyl bromides and subsequent exchange of bromide for tetrafluoroborate and trifluoroacetate anions. Non-modified and modified polymers bearing imidazole ligands inside the active centres were complexed with Cu(II) ions, leading to various copper content and applied as heterogeneous catalysts. The formed Cu(II) complexes were subsequently characterized by electron paramagnetic resonance (EPR) spectroscopy. A comparison of the EPR parameters, obtained by computer simulations of the experimental spectra of different Cu2+-catalysts allows to propose structures of the Cu(II) active centres as mostly N2O2 type (similar to the enzymatic ones). The catalytic activity of the Cu(II) loaded polymers without and with ionic liquids analogues inside active centres was tested using hydrogen peroxide in model oxidation reaction of hydroquinone. Protonation of imidazole nitrogen and introduction of additional free groups possessing strong electrostatic properties resulted in significant increase of catalytic activity in comparison to non-modified samples (oxidation degrees from 50 % to 90 %, selectivity up to 100 %). Trifluoroacetic anion has stronger effect than other studied anions.
Similar content being viewed by others
Introduction
Oxidoreductases (redox enzymes) are one of the most important classes of enzymes found in living systems. They can be found in all organisms: microbes, plants, animals. This extremely wide group of enzymes is essential to the life maintenance due to their physiological functions in the respiratory and energy-transducting processes [1]. The oxidoreductases catalyze the electrons or redox equivalents transfer between one substance and another, wherein these reactions proceed via simple electron transfer, hydrogen extraction, oxygen insertion etc. [2]. A variety of these mechanisms is reflected in oxidoreductases classification [3]. Three main subclasses may be distinguished: oxidases, oxygenases and peroxidases considering the common feature: oxygen atom as an electron acceptor. The oxidases and peroxidases react with oxygen from dioxygen molecule or hydrogen peroxide, respectively, and produce reactive intermediates for further reduction. The oxygenases catalyze insertion of one or two oxygen atoms from O2 to substrates [3]. Their active centres match the mechanism of action, which facilitates various modulation of redox potential, enzymes selectivity or reactivity towards groups of substrates. In all active centres of oxidoreductases common components can be distinguished: 1) metal ions/complexes (mostly Cu(II), Fe(III), Mn(II), Mo(III), V(V), Se(II), Ni(II)), 2) particular amino acids residues (histidine, cysteine, threonine etc.) and 3) coenzyme molecules e.g. FMN [2]. Together, these components enable to use catalytic properties of metal ions themselves and are responsible for the reaction processing inside the active centres shielded by polypeptide chain/s. The functional groups of amino acids in polymer matrix posses protecting as well as modulating functions. It is well known that they are responsible for the particular environment for reaction (hydrophilic, hydrophobic, ionic etc.), so these surrounding ligands can influence the reactivity, selectivity, redox-potential and stability of active centres.
The great importance of oxidoreductases in the nature, as well as their remarkable properties such as: ability of dioxygen activation during redox reactions, high redox potential, facile reaction control, extremely wide substrate range, high regio- and stereoselectivity, ability to introduce chirality to molecules, increasing hydrophilicity and polarity of reagents as well as great reaction acceleration under mild conditions result in the continuous increase of oxidation enzymes significance in chemical processes. Due to the biological properties and reactivity, compounds such as oxo- or hydroxy-type, appearing during catalysis, are important in pharmaceutical, food, agrochemical industries, chemical synthesis and environment protection. However, these catalysts are not perfect—there are several limitations on their applications such as low stability, sensitivity to external conditions (temperature, pH, solvents), complicated and time-consuming isolation and purification procedures etc. Hence, the enzymes inspire new research aimed at obtaining their synthetic mimics, based mainly on polymeric materials.
One of the most powerful method for the preparation of enzyme-like polymeric catalysts with artificial active centres able to recognize target molecules, is molecular imprinting (MI) [4]. This process involves: formation of the complex between template molecule and functional monomers, its subsequent polymerization in the presence of crosslinker and template removal after synthesis, which results in the creation of cavities in the polymer matrix (usually highly crosslinked), compatible in size, shape and functionality to the target molecule [5–8]. This approach enables to prepare materials exhibiting high selectivity and utilizing the same interactions as in biological systems, but having higher matrix rigidity and tolerance for external conditions. The details concerning the important parameters of the MI polymers syntheses were widely discussed in many reviews [5, 9–11]. One of the most important factor taken into account during design of biomimetic materials is a driving force applied for effective template binding to the functional groups of polymer chains e.g. covalent bonds, hydrogen bonds, metal coordination [4, 6, 12, 13]. Another important parameter is the choice of template molecule, dependent mainly on potential applications. For catalytic applications the use of transition state analoques (TSA) is suggested due to the best conditions offered for transition state stabilization during reaction [5, 14, 15]. Unfortunately, insufficient knowledge of the TSA resulted in application of the reaction reagents or their analogues as templates, which can cause catalysts substrate/product inhibition.
The catalytic activity of MI polymers is strongly related to the kinetics of reagents transport to/from the polymer matrix. Possible way to avoid diffusional problems during catalytic reaction and to facilitate reagents transport is to provide the conditions for the formation of recognition sites only at the inner surface of polymer matrix. This can be achieved by surface imprinting technique, in which W/O emulsion on organic-aqueous interface is utilized as a recognition field for template molecules to assure right orientation of the functional groups toward target molecules fixed at this interface [16–18]. This method is especially recommended for polymerization of more hydrophilic templates e.g. metal ions complexes.
Our studies focus on the creation of imprinted enzyme-like catalysts that mimics a large group of copper proteins, oxidases and oxygenases utilizing unique redox properties of copper ions. These interesting biocatalysts are acting in the presence of wide substrates range (phenols, amines, aromatic compounds, alcohols etc.) and the various Cu(II) active centres [19–21]. At the same time there are only few publications in which there enzymes were considered as the models for MI catalytic materials [22–24]. These enzymes usually contain active centres with Cu(II) ions coordinated by histidine ligands, water molecules and sulphur atoms from cysteine or methionine, as well as other amino acids residues (mainly glutamate, aspartate, threonine and phenylalanine) responsible for electron transfer and reagent binding (Fig. 1).
Ionic liquids (ILs) are ionic, salt-like materials that are liquid below 100 °C and consist of various ionic species (both organic and inorganic) giving billions of possible structures [25]. Figure 2 presents the most common ions used to create ILs, in which at least one ion has a delocalized charge and one component is bulky organic preventing the formation of a stable crystal lattice. The growing attention is given to ILs applications as green solvents because of their interesting properties as: excellent solvation qualities, variable viscosity range, wide temperature range together with negligible vapour pressure, excellent thermal and chemical stabilities, low combustibility, miscibility with water or organic solvents [26, 27]. The advantages such as high polarity, reactivity towards transition metal ions and ability to work in two-phase system, make these substances very useful and perspective for catalysis and polymer chemistry [28, 29]. Most of the published papers concerned the ILs applications as a reaction medium, significantly less papers focus on heterogenized ionic liquids and ILs as a polymerization medium [30–32].
The aim of the presented work was to synthesize surface molecularly imprinted polymers with Cu(II) ion in the active centres designed as in the model histydyl centres found in copper-oxidases and to modify the active centres to introduce the ionic liquids analogues, which are expected to improve catalytic activity of prepared materials. After basic characterization the obtained catalysts were tested in model reaction of hydroquinone oxidation using hydrogen peroxide.
Experimental
Materials
Most of the specified organic materials were purchased from Sigma-Aldrich except: 2,2′-azobis(isobutyronitrile) (AIBN)—Merck-Schuchardt and poly(vinyl alcohol)—Toyo Chemicals (Osaka, Japan). All inorganic materials were purchased from POCh Gliwice (Poland). 1-vinylimidazole was purified by distillation prior to use. Other chemicals and solvents were of analytical grade and used as obtained.
Synthesis of surface molecularly imprinted catalysts
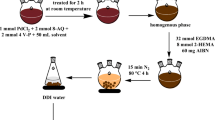
Starting surface imprinted polymers were prepared by suspension polymerization of W/O emulsion system used as a polymerization organic phase. First, the monomer mixture of three main monomers: 1-vinylimidazole (5 wt.%), 1,1,1-tris(hydroxymethyl)propane trimethacrylate (TMPMA, 40 wt.%), acrylonitrile (AN, 53.4 wt.%) and surface active monomer 4-vinyloxybutylstearate (VOBS; 1.6 wt.%) with template molecule 4-methoxybenzyl alcohol (MBA) was prepared in cyclohexanol. The diluent to monomers ratio was 1.8. Then, after adding 1 g of AIBN initiator this organic phase was added to aqueous phase of Cu(II) ions (0.003 mol) in acetate buffer (pH 4.7). The volume ratio of “water” and “oil” phases (W:O) was 1:2. Prepared mixture was sonicated for 20–40 s in various pulsing mode (4 s/3 s and 2 s/2 s, where s - seconds) using ultrasonic processor Sonics VCX-500 with standard probe. Obtained stable W/O emulsion was suspended in second aqueous phase consisting of 0.1 wt.% of poly(vinyl alcohol), 0.5 wt.% of additional surfactant—sodium dodecylsulfate (SDS) and 3 wt.% of sodium chloride. The polymerization was carried out under a flow of nitrogen at temperature ranged from 60 °C to 95 °C for 10 h with stirring at 180–220 rpm. The temperature was changed in given time intervals: 60 °C for 1 h, 70 °C for 1 h, 85 °C for 2 h and 95 °C for 6 h. Obtained polymer beads were washed with hot distilled water and acetone. After packing into column washing process was continued with anhydrous ethanol, distilled water and 1 M HCl solution repeatedly until colourless solution was seen.
Modification to ionic liquid analogues ligands
The obtained polymers were modified in a two step reaction (Fig. 3). In the first step, bromoalkylation on imidazole nitrogen was carried out using four alkyl bromides: 1-bromoethane (BE), 1-bromobutane (BB), 1-bromopentane (BP) and 1-bromohexane (BH). 5–8 g of imprinted MI polymers was swollen in 20–30 g of dioxane in the round-bottom flask, then the alkyl bromide was added in the 1:2 M ratio to imidazole ligand (IM). All reactions, except with 1-bromoethane, were carried out at dioxane boiling temperature under reflux for 24 h, because of adequately high boiling temperature of alkyl bromides in comparison to solvent. In case of BE the reaction temperature was kept below 40 °C to prevent reagent evaporation. Polymer samples after bromoalkylation were washed with dioxane, packed into columns and washed with distilled water.
In the second modification step, ion-exchange of bromide was done. Samples packed into columns were washed with an excess of 0.1 M solutions of sodium tetrafluoroborate or trifluoroacetic acid and subsequently with distilled water. After this process, the polymers were left to dry.
Physico-chemical characterization
The water regain W (g/g) and dioxane regain D (g/g) were measured using centrifugation technique (5 min, 3,000 rpm). The nitrogen content (N) was determined by modified Kjeldahl method and the theoretical nitrogen concentration (NT) was calculated from monomer mixture composition.
Concentration of imidazole ligands (ZIM) expressed as a strongly basic amino groups concentration was determined using ion-exchange process on these groups. In this method the polymer samples packed in columns were washed with 0.001 M HCl to pH 4 to introduce chloride anions on amine. Afterwards, samples were washed with 200 mL of 4 wt.% solution of sodium sulphate to exchange chlorides for sulphate anions – filtrates were collected and diluted with distilled water to 250 mL. Chloride concentration in obtained solutions was subsequently determined by the typical Volhard method.
Bromide concentration (ZBr) after bromoalkylation was analysed using Schöniger’s flask test involving sample combustion in pure oxygen, followed by absorption of the combustion products by 5 % hydrogen peroxide solution. Absorbed bromide concentration in solutions was subsequently determined by the Volhard method.
The FT-IR spectroscopic analysis was performed using a Perkin-Elmer 1600 FT-IR spectrometer. Dried samples were first grated and then prepared as discs with KBr.
The scanning electron microscopy (SEM) was carried out using a Zeiss EVO microscope. All samples were coated with gold prior to imaging.
The surface area measurements were performed using Micromeritics ASAP 2020 nitrogen sorption porosimeter. All samples before analysis were degassed for 12 h under vacuum at 50 °C. Physisorption of nitrogen was carried out at liquid nitrogen temperature.
Catalysts preparation
Sorption of Cu(II) ions was performed using batch method [33, 34]. All resins, swollen in distilled water, were placed in 10 to 25 ml of copper(II) acetate solutions (5 × 10−4 and 5 × 10−3 M) in acetate buffer pH 5.0. Samples were shaken at room temperature for 48 h and then separated from the solutions. The Cu(II) concentration in solution was determined by atomic absorption spectrophotometry (AAS) using GBC Avanta spectrometer. Sorption of copper S (μmol/g) was calculated from the difference of Cu(II) concentration in solution before and after sorption.
Catalysts characterization
EPR measurements
The EPR spectra were measured using a Bruker Elexsys E500 spectrometer equipped with NMR teslameter (ER 036TM) and frequency counter (E 41 FC) at X-band. The simulations of the experimental spectra were performed using computer program WINEPR Simfonia, version 1.26, and the program written by Dr Andrew Ozarowski from NHMFL, University of Florida, with resonance field calculated by diagonalization of energy matrix.
Model catalysis reaction
Catalysts were placed in polyethylene flasks and swollen in 10 ml hydrogen peroxide solution (4.8–5.3 × 10−2 M in acetate buffer pH 5.0). After 30 min the mixtures were purged with gaseous nitrogen and then 10 ml of 4 × 10−3 M hydroquinone (H2Q) was added [33]. The Cu(II) to substrate ratio was 1:10. The entire mixture was shaken (350 cycles/min) in water bath at 35 °C (H2Q) for 80 min. Samples were taken at given time interval and were 10-fold diluted with distilled water for further analysis. Additionally, reaction without catalyst and with non-loaded polymeric samples after modifications were investigated. The concentrations of unreacted substrate and the product, p-benzoquinone (Q), were determined by UV–vis spectrophotometry using Jasco V-630 spectrophotometer at λ = 289 nm and λ = 246 nm, respectively. The oxidation degree of substrate (LS; %) and yield of product (YP; %) were defined as a decrease of substrate concentration and an increase of product concentration, respectively, with relation to the initial substrate concentration. Selectivity of product (SP; %) was defined as a ratio of product concentration to the reacted substrate concentration.
Results and discussion
Synthesis and characteristic of starting polymers and modified resins
Three starting materials imprinted with Cu(II) ions and template molecule were prepared by suspension polymerization of W/O emulsion using the same mixture composition in all syntheses. Designed W/O/W system, presented in Fig. 4, enabled to create cavities, which are complementary to reaction reagents directly on the polymer surface. As it was described previously [35] such placement of active centres is advantageous for reagents diffusion and reaction selectivity. Besides Cu(II) ions, to create catalytically active cites in the polymer matrix structural analogue of reaction reagents, 4-methoxybenzyl alcohol has been chosen as the template. This choice was dictated by the formation of interactions between metal ions, polymer functional ligands and hydroxyl group of MBA similar to this found in natural enzymes during catalysis. Additionally, complexes created with monodentate imidazole ligands showed relatively weak stability, which makes them easy to remove from polymer matrix using simple washing with hydrochloric acid.
As a main functional monomer able to create Cu(II) active centres, 1-vinylimidazole has been chosen. It is widely accepted as a histydyl ligand analogue despite the fact that imidazole ring binding to the polymer chain is different than in the nature (binding through nitrogen instead of carbon). Additionally, application of this monomer allowed for further modification of imidazole to ionic liquids analogues. The use of flexible multifunctional crosslinker - TMPMA guaranteed porous, moderately rigid structure of the supports with pores complementary to the templating complex shape characterized by higher hydrophilicity than in the copolymers with traditionally used DVB crosslinker [36]. The parameters as rigidity, flexibility, hydrophilicity were widely discussed as essential for the preparation and applications of imprinting materials [4, 10]. Addition of acrylonitrile (AN) to monomers mixture was aimed at preservation of the rigid, porous structure without the necessity of using high crosslinker amounts. To increase the stability of W/O emulsion during synthesis and to facilitate metal ions removal after polymerization small amount of surface active monomer 4-vinyloxybutylstearate (VOBS) was used.
The presence of all mers in the imprinted polymers was confirmed using FT-IR method. The FT-IR spectra for one line of starting and modified polymers shown in Fig. 5 are practically the same. In the spectrum of polymer before any chemical modification the peaks confirming the presence of functional groups originating from monomers used in polymerization can be clearly seen. In all samples two strong peaks ascribed to the C(O)-O group of TMPMA were observed at about: 1,734 cm−1 (C = O vibration) and 1,226 cm−1 (C-O). The band typical for nitrile C ≡ N group of AN was present at 2,242 cm−1. In all spectra several bands assignable to imidazole ligand may be distinguished. In 3,600–2,990 cm−1 region two typical peaks could be observed: ν(NH) stretching band at 3,360–3,450 cm−1 (consisting of two or three subbands) and ν(CH) band at 2,945–2,975 cm−1. Small shift of the bands characteristic for imidazole aromatic ring: the band for imidazole ring stretching observed at 1,635 cm−1 and the (C = N) and/or (CH = CH) stretching vibrations at 1,470 cm−1 confirmed the presence of Cu(II)-VI complex during polymerization. In the region of 820–740 cm−1 peaks characteristic for aromatic C-H deformation vibration of imidazole ring appeared. Upon the alkylation and then anion exchange the spectra do not change much and only small shifts in the positions of peaks corresponding to the imidazole are observed. In any case the FTIR spectra of solid samples are not suitable for even semiquantitative analysis but the results are further supported by the bromide content analysis. Only in case of CF3COO− very small peak at 720 cm−1 could be observed.
FT-IR spectra of chosen samples from one polymer line: a non-modified surface imprinted terpolymer VI/AN/TMPMA—M1s; b, c modified surface MIP after bromoalkylation with 1-bromobutane and bromide exchange for BF4 − (M1s_mod2a) and for CF3COO− (M1s_mod2b), respectively; d modified surface MIP after bromoalkylation with 1-bromohexane and bromide exchange for BF4 − (M1s_mod4a)
The characteristics of obtained supports are presented in Table 1. Considering the same mixture composition, the main physicochemical parameters for all polymers depended on sonication mode. Resin synthesized from W/O emulsion obtained using longer pulsing mode (4 s/3 s) varied widely from the rest of samples, while the resins prepared using the same pulsing mode (2 s/2 s) in the first step of the synthesis showed similar parameters. In all resins the nitrogen concentration was much lower than nominal value calculated from monomer mixture composition (from 14 % to 34 % of theoretical nitrogen content), which was probably the result of a small yield of AN incorporation to the polymeric resin during suspension polymerization due to the high solubility of acrylonitrile in aqueous solutions or formation of linear polyacrylonitrile chains subsequently extracted after polymerization [37]. Another reason of nitrogen content diminution was the inhibiting effect of metal ions on the radical mechanism of polymerization. Both effects had stronger influence on the parameters for M1s/I. Sonication mode with longer breaks between ultrasonic pulses resulted in larger droplets and slightly less stable emulsion, which influenced the imprinting/polymerization process.
Also the concentration of imidazole ligands varied between particular samples, which could be ascribed to formation of polyvinylimidazole homopolymer and its partial solubility in water dependent on emulsion stability. The decrease of imidazole content caused reduction of bromide content after first step of modification. The Br to IM ratio was established as 1:2, to ensure the substitution on approximately half of nitrogen atoms, which was confirmed by bromide analysis results. The Br− content in modified samples M1s/II and M1s/III was below the assay quantification limit due to the very small amount of IM ligands.
As can be seen from the Table 1, all samples were porous due to the presence of 40 wt.% of crosslinker and an excess of solvent to monomers in polymerization mixture. The scanning electron microscopy (SEM) was used to examine the morphology and size of polymer beads. The SEM images of all starting materials and modified samples are shown in Fig. 6. These resins were rigid, regular spheres exhibiting size distribution polydispersity. The particle diameter of sample M1s/I was ranged from 50 μm to 300 μm (Fig. 6a and d), while of samples M1s/II and III ranged from 30 μm to 200 μm (Fig. 6b and c). The surfaces shown on micrographs in Fig. 6e and f were rough and rugged typically for porous materials. The reduction of pulsing mode led to changes in surface morphology: increase of its homogeneity and smoothness. There is no visible changes in surface morphology after modifications.
The observed surface characteristics found confirmation in the results of nitrogen physisorption porosimetry (Table 1). The specific surface as well as pore volume and average pore diameter were calculated using Brunauer-Emmett-Teller (BET) isotherm. It could be seen that all polymers were moderately porous with specific surface area between 79 m2/g for M1s/III sample to 315 m2/g for M1s/I depending on pulsing mode. The values of average pore diameters between 5.2 nm and 15.6 nm pointed out the presence of pores mainly in mesoporous range. According to the principles of the surface imprinting technique, emulsion properties influenced mostly the meso/macroporous polymer structure and only a little the microporous one, which could find confirmation in the results of mean micropore diameter similar for all samples.
Catalysts characterization
After polymer synthesis the Cu(II)-template complexes were removed to obtain “empty” resins ready to further modifications. Samples with ionic liquids analogues, as well as non-modified ones were subsequently loaded with Cu(II) ions to prepare catalysts with quantified active centres. Under applied conditions sorption led to the catalysts with Cu(II) loading ranged from 10 μmol/g to 150 μmol/g. Inside the active centres Cu(II) ions may coordinate imidazole nitrogen donor and/or oxygen donors from carboxyl group formed as the result of TMPMA hydrolysis during polymerization or from water molecule.
All the studied polymers loaded by Cu2+ ions exhibit EPR spectra with good resolution of the hyperfine splitting due to interaction between the spins of cooper nucleus (I = 3/2) and unpaired electron (S = 1/2) at magnetic field around g║ position (Fig. 7).
EPR spectra of Cu2+ complexes (at 77 K) formed with imidazole groups attached to the initial and modified polymers in comparison with the spectra simulated (sim) according to the parameters given in Table 3
The experimental spectra were computer simulated using the parameters which satisfy the relations g║>>g⊥ > 2.0023 and A║>>A⊥ (Table 2) corresponding to Cu2+ complexes of elongated octahedral geometries with dx2–y2 orbital of unpaired electron ground state.
Inspection of the EPR parameters collected in Table 2 suggests that Cu2+ complexes with g║ about 2.3 and A║ about 165 × 10−4 cm−1 are dominant suggesting involvement of two imidazole ligands in their in-plane coordination sphere in agreement with the EPR parameters observed for model Cu2+ complexes with substituted imidazole ligands [38, 39]. The remaining donor positions are occupied by oxygen atoms of water molecules resulting in N2O2 donor set. It is remarkable that the polymers modified, according to the reactions shown in Fig. 3 using 1-bromobutane exhibit preference to Cu(II) coordination with higher amount of imidazole ligands leading to the N3O coordination, although the spectra with the polymers modified by 1-bromoethane have apparently higher intensity indicating greater content of Cu(II) complexes. Presented EPR parameters allows to propose the possible structures of Cu(II) active centres (Fig. 8) showing similarity to the natural ones. Increase of catalysts’ Cu(II) loading enhances the spectra intensity without significant change of EPR parameters and suggested complexes structure, because of very small amount of imidazole ligand in comparison to metal content, accessible to coordination.
Previous investigations on enzyme-like catalysts indicated the great importance of complex environment formed by free ligands inside the active centres for catalytic activity of polymeric biomimetics [40]. The catalysts after sorption were preliminary tested in model oxidation reaction of hydroquinone (H2Q) to p-benzoquinone (Q) using hydrogen peroxide in aqueous medium (Fig. 9). Despite of possible further Q oxidation to 2,5-dihydroxy-p-benzoquinone (Q(OH)2) happening under disadvantageous conditions, this reaction is appropriate to characterize the basic catalytic properties of the tested samples due to mild reaction conditions, short time and simple measurement procedure. Details concerning the UV–vis spectra parameters and catalytic oxidation were discussed earlier [31].
The catalytic activity of samples is strongly related to the presence of catalytic metal ion inside active sites. Both polymers: non-modified and modified containing heterogeneous ionic liquids analogues without Cu(II) ions, under the same reaction conditions showed very weak catalytic activity ascribed to the polymer functional groups (Table 3). The oxidation degree after 60 min ranged from 3.8 % for the catalysts modified with BP/CF3COO− to 56.1 % for BP/BF4 −. Only in case of modification with BP/BF4 −, quite high reactivity of non-loaded sample was observed what may indicate the positive influence of incorporated ILs analogue on catalytic activity. However, problems with diffusion of reagents to the active centres was noticed during reaction causing continuous reagents concentration fluctuations. In comparison to the non-catalyzed reaction, some of the catalyst without Cu(II) ions are not active in hydroquinone oxidation—the similar values of catalytic parameters are related to the self activity of the substrate.
The results of hydroquinone oxidation are given in Figs. 10 and 11. Generally, the modification inside the active centres giving free charged groups around the catalytic copper complex improved catalytic activity of tested samples relating to non-modified sample with similar Cu(II) loading. However, this improvement varied with the anion type and N-substituent. Two samples: bromoalkylated with BE (mod1) and BP (mod3) showed significantly higher reactivity and selectivity, at the same time the other samples showed an increase of only reaction selectivity (up to 100 %). In the tested reaction the worst catalysts were both bromoalkylated ILs analogues having different complex type (N3O) inside the active centres. The rest of the samples formed N2O2 type Cu(II) complexes, known as the most active in catalysis. Considering the disproportion between oxidation degree and selectivity, the best catalyst—modified with BE might be pointed out. Despite of slightly lower reaction parameters than for BP (LS and SP) and BH (SP), this catalyst achieved the less variable results through the total duration of the oxidation. Unfortunately, there is no clear correlation between the length of alkyl substituent on imidazole nitrogen and the activity of samples.
Comparing the results for both anions of ILs analogues one could observed that better anion is trifluoroacetate (higher oxidation degrees). This is probably related to the strong inductive effect of fluorine atoms on carboxylic group and increase of its acid strength influencing the Cu2+ coordination sphere. Additionally, this strongly acidic anion can better stabilize the reagents during reactions. The greater effect of tetrafluoroborate anion on reaction selectivity, in spite of his worse electrostatic properties, may be associated with the anion size giving smaller steric hindrance inside the active centres.
It was discussed previously that the decrease of Cu(II) loading improved catalytic activity of biomimetic catalysts due to the great importance of isolation of active centres. This phenomenon was partly neutralized by the positive effect of both anions on protonated nitrogen (Table 4).
Conclusions
Catalysts prepared using surface molecularly imprinting technique with Cu(II) ions and 4-methoxybenzyl alcohol as the template may be successfully modified to create active oxidation enzymes mimics. Use of surface imprinting technique ensures effective diffusion to/from active centres and high catalytic activity of Cu(II) ions complexes of N2O2 type inside active sites. Protonation of imidazole nitrogen during bromoalkylation reactions and further anion exchange for anions with stronger electrostatic properties: tetrafluoroborate and trifluoroacetate result in significant increase of catalytic properties in comparison to non-modified sample. Obtained modified catalysts have a great potential for further exploitation due to the positive effect of ion liquids analogues heterogenized inside the active centres on catalyzed reaction. Additionally, an introduction of free active groups to the active centres make the dependence between metal loading and complex activity less important.
References
Munro AW, Taylor P, Walkinshaw MD (2000) Curr Opin Biotechnol 11:369–76
Xu F (2005) Industr Biotechnol 1:38–50
Burton SC (2001) Trends Biotechnol 21:543–9
Wulff G, Sarhan A (1972) Angew Chem Int Ed Engl 11:341–4
Wulff G (2002) Chem Rev 102:1–27
Sellergren B (2001) Molecularly imprinted polymers—man-made mimics of antibodies and their application in analytical chemistry. Elsevier, Amsterdam
Haupt K, Mosbach K (2000) Chem Rev 100:2495–504
Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, O’Mahony J, Whitcombe MJ (2006) J Mol Recog 19:106–80
Sellergren B (1999) Trends Anal Chem 18:164–74
Cormack PAG, Elorza AZ (2004) J Chromatogr B 804:173–82
Vasapollo G, Del Sole R, Mergola L, Lazzoi MR, Scardino A, Scorrano S, Mele G (2011) Int J Mol Sci 12:5908–45
Severin K (2000) Curr Opin Chem Biol 4:710–4
Striegler S (2004) J Chromatogr B 804:183–95
Motherwell WB, Bingham MJ, Six Y (2004) Tetrahedron 57:4663–86
Wulff G, Liu J (2012) ACS Acc Chem Res 45:239–47
Yoshida M, Uezu K, Goto M, Furusaki S (1999) J Appl Polym Sci 73:1223–30
Yoshida M, Hatate Y, Uezu K, Goto M, Furusaki S (2000) Colloid Surf A 169:259–69
Toorisaka E, Uezu K, Goto M, Furusaki S (2003) Biochem Engeen J 14:85–91
Mukherjee S, Basak B, Bhunia B, Dey A, Mondal B (2013) Rev Environ Sci Biotechnol 12:61–73
Duran N, Rosa MA, D’Annibale A, Gianfreda L (2002) Enzym Microb Technol 31:907–31
Enguita FJ, Marçal D, Martins LO, Grenha R, Henriques AO, Lindley PF, Carrondo MA (2004) J Biol Chem 279:23472–6
Zhukhlistova NE, Zhukova YN, Lyashenko AV, Zaitsev VN, Mikhailov (2008) Crystallogr Rep 53:92–109
Sergeyeva TA, Slinchenko OA, Gorbach LA, Matyushov VF, Brovko OO, Piletsky SA, Sergeeva LM, Elska GV (2010) Anal Chim Acta 659:274–9
Piletsky SA, Nicholls IA, Rozhko MI, Sergeyeva TA, Piletska EV, El’skaya AV, Karube I (2005) Ukr Biochem J 77:63–7
Cooper ER, Andrews CD, Wheatley PS, Webb PB, Wormland P, Morris RE (2004) Nature 430:1012–6
Freemantle M (2010) An introduction to ionic liquids. Royal Society of Chemistry Publishing, Cambridge, UK
Dupont J, de Souza RF, Suarez PAZ (2002) Chem Rev 102:3667–91
Wasserscheid P, Welton T (2008) Ionic liquids in synthesis. Wiley-VCH, Weinheim, Germany
Mehnert CP (2005) Chem Eur J 11:50–6
Kubisa P (2004) Prog Polym Sci 29:3–12
Welton T (2004) Coord Chem Rev 248:2459–77
Olivier-Bourbigou H, Magna L (2002) J Mol Catal A Chem 182–183:419–37
Owsik I, Kolarz BN (2002) J Mol Catal A Chem 178:63–71
Jakubiak A, Owsik IA, Kolarz BN (2005) React Funct Polym 65:161–7
Jakubiak A, Kolarz B, Jezierska J (2006) Macromol Symp 235:127–35
Kolarz BN, Jermakowicz-Bartkowiak D, Trochimczuk A (1997) Eur Polym J 34:1191–7
Riqueza EC, Aguiar AP, Santa Maria LC, Aguiar MRMP (2002) Polym Bull 48:407–14
Kurdziel K, Głowiak T, Jezierska J (2000) J Chem Soc Dalton Trans 7:1095–1100
Kurdziel K, Głowiak T, Jezierska J (2001) Polyhedron 20:3307–13
Owsik IA, Kolarz BN (2004) Catal Today 91–92:109–204
Acknowledgment
This work was supported by Polish Ministry of Science and Higher Education Grant No. N N204 137238.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Jakubiak-Marcinkowska, A., Legan, M. & Jezierska, J. Molecularly imprinted polymeric Cu(II) catalysts with modified active centres mimicking oxidation enzymes. J Polym Res 20, 317 (2013). https://doi.org/10.1007/s10965-013-0317-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0317-z