Abstract
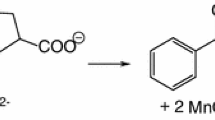
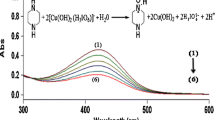
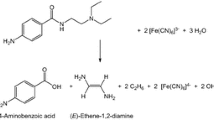
The oxidation of ketorolac (KET) by diperiodatocuprate(III) (DPC) in aqueous alkaline medium at a constant ionic strength of 0.10 mol⋅dm−3 was studied spectrophotometrically at 298 K. The reaction is of first order in [DPC] and has less than unit order in both [KET] and [alkali], and negative fractional order in [periodate]. The oxidation reaction in alkaline medium has been shown to proceed via a DPC-ketorolac complex, which decomposes slowly in a rate determining step followed by other fast steps to give the products. The main products were identified by spot test, IR and GC-MS spectral studies. The reaction constants involved in the different steps of the mechanism were calculated at different temperatures, which yielded thermodynamic quantities for different steps of the reaction scheme. The activation parameters with respect to the slow step of the mechanism were computed and discussed; thermodynamic quantities were also determined.
Similar content being viewed by others
References
Murray, M.D., Brater, D.C.: Renal toxicity of the non-steroidal anti-inflammatory drugs. Annu. Rev. Pharmacol. Toxicol. 33, 435–465 (1993)
Alsarra, I.A., Bosela, A.A., Ahmed, S.M., Mahrous, G.M.: Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur. J. Pharm. Biopharm. 59, 485–490 (2005)
Reddy, K.B., Sethuram, B., Navaneeth Rao, T.: Kinetics of benzaldehydes by copper(III) in tert- butanol–water medium. Indian J. Chem. 23A, 593–595 (1984)
Kumar, A., Kumar, P., Ramamurthy, P.: Kinetics of oxidation of glycine and related substrates by diperiodatoargentate(III). Polyhedron 18, 773–780 (1999)
Ali Khan, J., Chandaiah, U., Kishore Kumar, B., Kadlikar, S.: Kinetics of electron transfer from cyclic ketones to Ni(IV) periodate complex in aqueous alkaline medium. Bull. Chem. Soc. Jpn. 62, 1300–1303 (1989)
Niu, W., Zhu, Y., Hu, K., Tong, C., Yang, H.: Kinetics of oxidation of SCN− by diperiodatocuprate(III) (DPC) in alkaline medium. Int. J. Chem. Kinet. 28, 899–904 (1996)
RamReddy, M.G., Sethuram, B., Navaneeth Rao, T.: Effect of copper(I) catalysed oxidation of some amino acids by peroxydisulphate ion in aqueous alkaline medium. Indian J. Chem. 16A, 313–316 (1978)
Karlin, K.D., Gultneh, Y.: Progress in Inorganic Chemistry. Lipard, S.J. (ed.) Wiley, New York (1997)
Jose, T., Tuwar, S.M.: Oxidation of threonine by the analytical reagent diperiodatocuprate(III) an autocatalysed reaction. J. Mol. Struct. 827, 137–144 (2007)
Karlin, K.D., Kaderli, S., Zuberbuhler, A.D.: Kinetics and thermodynamics of copper(I)/dioxygen interaction. Acc. Chem. Res. 30, 139–147 (1997)
Sethuram, B.: Some aspects of Electron Transfer Reactions Involving Organic Molecules, p. 73. Allied Publishers (P) Ltd., New Delhi (2003)
Shetti, N.P., Nandibewoor, S.T.: Kinetic and mechanistic investigations on oxidation of L-tryptophan by diperiodatocuprate(III) in aqueous alkaline medium. Z. Phys. Chem. 223, 299–317 (2009)
Murthy, C.P., Sethuram, B., Navaneeth Rao, T.: Kinetics of oxidation of some alcohols by diperiodatocuprate(III) in alkaline medium. Z. Phys. Chem. 262, 336–340 (1981)
Jeffery, G.H., Bassett, J., Mendham, J., Denney, R.C.: Vogel’s Textbook of Quantitative Chemical Analysis, ELBS, p. 455. Longman, Essex (1996)
Panigrahi, G.P., Misro, P.K.: Kinetics and mechanism of oxidation of aliphatic ketones by sodium metaperiodate: A comparative study of uncatalysed versus osmium tetraoxide- catalysed oxidation. Indian J. Chem. 16A, 762–766 (1978)
Hiremath, G.C., Mulla, R.M., Nandibewoor, S.T.: Mechanistic study of the oxidation of isonicotinate ion by diperiodatocuprate(III) in aqueous alkaline medium. J. Chem. Res. 2005, 197–201 (2005)
Kolthoff, I.M., Meehan, E.J., Carr, E.M.: Mechanism of initiation of emulsion polymerization by persulfate. J. Am. Chem. Soc. 75, 1439–1441 (1953)
Reddy, K.B., Sethuram, B., Navaneeth Rao, T.: Photon cross sections in copper, platinium and gold at 81 Kev. Z. Phys. Chem. 268, 706–710 (1987)
Bailar, J.C., Jr., Emeleus, H.J., Nyholm, S.R., Trotman-Dikenson, A.F.: Comprehensive Inorganic Chemistry, p. 1456. Pergamon, Oxford (1975)
Naik, P.N., Kulkarni, S.D., Chimatadar, S.A., Nandibewoor, S.T.: Mechanistic study of oxidation of sulfacetamide by diperiodatocuprate(III) in aqueous alkaline medium. Indian J. Chem. 47A, 1666–1670 (2008)
Rangappa, K.S., Raghavendra, M.P., Mahadevappa, D.S., Channegouda, D.: Sodium n-chlorobenzenesulfonamide as a selective oxidant for hexosamines in alkaline medium: a kinetic and mechanistic study. J. Org. Chem. 63, 531–536 (1998)
Weissberger, A., Lewis, E.S. (eds.): Investigations of Rates and Mechanism of Reactions in Techniques of Chemistry, p. 421. Wiley, New York (1974)
Hiremath, D.C., Sirsalmath, K.T., Nandibewoor, S.T.: Osmium(VIII)/ruthenium(III)-catalysed oxidation of L-lysine by diperiodatocuprate(III) in aqueous alkaline medium—a comparative mechanistic approach by stopped flow technique. Catal Lett. 122, 144–154 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malode, S.J., Shetti, N.P. & Nandibewoor, S.T. Thermodynamic Quantities for the Different Steps Involved in the Oxidation of the Drug Ketorolac by Copper(III) Periodate Complex in Aqueous Alkaline Medium: A Mechanistic Approach. J Solution Chem 39, 417–430 (2010). https://doi.org/10.1007/s10953-010-9501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-010-9501-5