Abstract
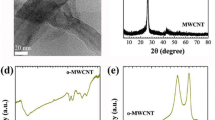
We prepared three ferrite nanocatalysts: (i) copper ferrite (CuFe2O4) (ii) ferrite where cobalt was substituted by nickel (Ni x Co1−x Fe2O4, with x=0, 0.2, 0.4, 0.6), and (iii) ferrite where nickel was substituted by zinc (Zn y Ni1−y Fe2O4 with y=1, 0.7, 0.5, 0.3), by the sol-gel method. The X-ray diffraction patterns show that the ferrite samples have been crystallized in the cubic spinel structural phase. We obtained the size of grains by field emission scanning electron microscopy images and their magnetic properties by vibrating sample magnetometer. Next, carbon nanotubes were grown on these nanocatalysts by the catalytic chemical vapor deposition method. We show that the catalytic activity of these nanocrystals on the carbon nanotube growth depend on cation distributions in the octahedral and tetrahedral sites, structural isotropy, and catalytic activity due to cations. Our study may have applications in finding a suitable candidate of doped ferrite nanocrystals as catalysts for carbon nanotube growth. More interestingly, the yield of fabrication of carbon nanotubes can be considered as an indirect tool to study catalytic activity of ferrites.




Similar content being viewed by others
References
Bersuker, I.B.: Electronic Structure and Properties of Transition Metal Compounds: Introduction to the Theory. Wiley, New York (1996)
Burns, R.: Mineralogical Applications of Crystal Field Theory, vol. 5. Cambridge University Press, Cambridge (1993)
Borg, R.J., Dienes, G.J.: Physical Chemistry of Solids. Academic Press, San Diego (1992)
El-Sayed, A.M.: Electrical conductivity of nickel–zinc and Cr substituted nickel–zinc ferrites. Mater. Chem. Phys. 82, 583 (2003)
Goldman, A.: Modem Ferrite Technology, 2nd edn., pp. 63–71. Springer, Pittsburgh (1987). ISBN 10: 0-387-29413-9
Ferreira, T.A.S., Waerenborgh, J.C., Mendonca, M.H.R.M., Nunes, M.R., Costa, F.M.: Structural and morphological characterization of FeCo2O4 and CoFe2O4 spinels prepared by a coprecipitation method. Solid State Sci. 5, 383 (2003)
Murray, P.J., Linnette, J.W.: Cation distribution in the spinels Co x Fe3−x O4. J. Phys. Chem. Solids 37, 1041 (1976)
Lahiri, P., Sengupta, S.K.: Spinel ferrites as catalyst: a study on catalytic effect of coprecipitated ferrites on hydrogen peroxide decomposition. Can. J. Chem. 69, 33 (1991)
Goldstein, J.R., Tseung, A.C.C.: The kinetics of hydrogen peroxide decomposition catalyzed by cobalt-iron oxides. J. Catal. 32, 452 (1974)
Krishnamurthy, K.R., Viswanathan, B., Sastri, M.V.C.: Catalytic activity of transition metal spinel type ferrites: structure-activity correlations in the oxidation of CO. J. Res. Inst. Catal, Hokkaido Univ. 24, 219 (1976)
Massoth, F.E., Scarpiello, P.A.: Catalyst characterization studies on the Zn–Cr–Fe oxide system. J. Catal. 21, 294 (1971)
Néel, L.: Magnetic properties of femtes: ferrimagnetism and antiferromagnetism. Ann. Phys. Paris 3, 137 (1948)
Belin, T., Epron, F.: Characterization methods of carbon nanotubes: a review. Mater. Sci. Eng. B, Solid-State Mater. Adv. Technol. 119, 105 (2005)
Lambin, P., Loiseau, A., Culot, C., Biro, L.: Structure of carbon nanotubes probed by local and global probes. Carbon 40, 1635 (2002)
Liu, M., Cowley, J.: Structures of the helical carbon nanotubes. Carbon 32, 393 (1994)
Bernaerts, D., Amelinckx, S., Lambin, P., Lucas, A.: The diffraction space of circular and polygonized multishell nanotubules. Appl. Phys. A 67, 53 (1998)
Figueiredo, J.L., Orfao, J.J.M., Cunha, A.F.: Hydrogen production via methane decomposition on Raney-type catalysts. Int. J. Hydrog. Energy 35, 9795 (2010)
Acknowledgements
The authors acknowledge the Iranian Nano Technology Initiative Council and Vice Chair for research of Alzahra University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini Akbarnejad, R., Daadmehr, V., Rezakhani, A.T. et al. Catalytic Activity of the Spinel Ferrite Nanocrystals on the Growth of Carbon Nanotubes. J Supercond Nov Magn 26, 429–435 (2013). https://doi.org/10.1007/s10948-012-1758-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1758-z