Abstract
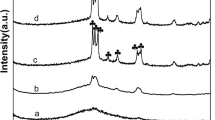
A series of vanadium substituted phosphomolybdic acid (VPMA) supported on mesoporous MCM-41 catalysts with varying VPMA content ranging from 10 to 50 wt% were prepared by impregnation method. The samples were characterized by X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy, Raman spectroscopy, scanning electron microscopy, N2 adsorption–desorption measurements to determine surface area and pore size distribution. The acidity measurements were studied by temperature programmed desorption (TPD) of NH3 and nature of acidic sites were examined by pyridine adsorbed FT-IR spectra. XRD results and N2 adsorption–desorption isotherms revealed the retention of ordered mesoporous structure of MCM-41 and the uniform pore structure with increase in HPA loading. FT-IR and Raman spectra showed that the primary structure of the Keggin units of the VPMA remains intact with the MCM-41. The TPD-NH3 showed that acidity of the catalysts increased with increase of VPMA loading. The findings of FT-IR spectra of pyridine adsorption revealed that VPMA/MCM-41 catalysts contain both the Brønsted and Lewis acidic sites and the amount of Brønsted acidic sites increased with increase of VPMA loading up to 40 wt% on the support. The catalysts were tested for the vapour phase dehydration of glycerol to acrolein. The catalyst samples were found to be highly active with 100% conversion and the acrolein selectivity changed with VPMA active phase loading on the support. In summary, the catalytic properties in terms of conversion and selectivity are attributed to the acidity, structural and textural properties of VPMA/MCM-41 catalyst.











Similar content being viewed by others
References
T.R. Gaydhankar, V. Samuel, P.N. Joshi, Mater. Lett. 60, 957–961 (2006)
V. Brahmkhatri, A. Patel, Fuel 102, 72–77 (2012)
A.E.R.S. Khder, H.M.A. Hassan, M.S.E. Shall, Appl. Catal A 77, 411–412 (2012)
A.M. Alsalme, P.V. Wiper, Y.Z. Khimyak, E.F. Kozhevnikova, I.V. Kozhevnikov, J. Catal. 276, 181–189 (2010)
R.S. Weber, J. Phys. Chem. 98, 2999–3005 (1994)
M. Kanno, Y.K. Miura, T. Yasukawa, T. Hasegawa, W. Ninomiya, K. Ooyachi, H. Imai, T. Tatsumi, Y. Kamiya, Catal. Commun. 13, 59–62 (2011)
S. Benadji, P. Eloy, A. Léonard, B.L. Su, K. Bachari, C. Rabia, E.M. Gaigneaux, Microporous Mesoporous Mater. 130, 103–114 (2010)
J. Shan, Z. Li, S. Zhu, H. Liu, J. Li, J. Wang, W. Fan, Catalysts 121, 1–15 (2019)
J. Ding, T. Ma, R. Shao, W. Xu, P. Wang, X. Song, R. Guan, K. Yeung, W. Han, New J. Chem. 42, 14271–14280 (2018)
B. Viswanadham, A. Srikanth, V.P. Kumar, K.V.R. Chary, J. Nanosci. Nanotechnol. 15, 5391–5402 (2015)
B. Viswanadham, N. Nekkala, C.N. Rohitha, V. Vishwanathan, K.V.R. Chary, Catal. Lett. 148, 397–406 (2018)
G. Karthikeyan, A. Pandurangan, J. Mol. Catal. A 311, 36–45 (2009)
M. Kanno, T. Yasukawa, W. Ninomiya, K. Ooyachi, Y. Kamiya, J. Catal. 273, 1–8 (2010)
M.S. Kaba, M.A. Barteau, W.Y. Lee, I.K. Song, Appl. Catal. A 129, 194–195 (2000)
J. Zhang, Y. Tang, G. Li, C. Hu, Appl. Catal. A 278, 251–261 (2005)
K. Benjamin, P. Sebastien, B.B. Virginie, R. Patrick, D. Franck, Green Chem. 12, 2079–2098 (2010)
P.L. Garbay, J.M.M. Millet, S. Loridant, V.B. Baca, P. Rey, J. Catal. 280, 68–76 (2011)
Y. Gu, N. Cui, Q. Yu, C. Li, Q. Cui, Appl. Catal. A 9, 429–430 (2012)
M.A. Barteau, J.E. Lyons, I.K. Song, J. Catal. 216, 236–245 (2003)
L.Z. Tao, S.H. Chai, Y. Zuo, W.T. Zheng, Y. Liang, B.Q. Xu, Catal. Today 158, 310–316 (2010)
Y.T. Kim, K.D. Jung, E.D. Park, Appl. Catal. A 393, 275–287 (2011)
W. Suprun, M. Lutecki, T. Haber, H. Papp, J. Mol. Catal. A 309, 71–78 (2009)
M. Herbon, A. Lange, M. Wei, W. Suprun, D. Enke, Chem. Eng. Technol. 38, 431–440 (2015)
Acknowledgements
The authors thank Director, CSIR-IICT, Hyderabad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Viswanadham, B., Vishwanathan, V., Chary, K.V.R. et al. Catalytic dehydration of glycerol to acrolein over mesoporous MCM-41 supported heteropolyacid catalysts. J Porous Mater 28, 1269–1279 (2021). https://doi.org/10.1007/s10934-021-01070-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01070-8