Abstract
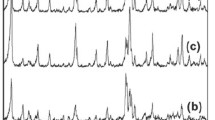
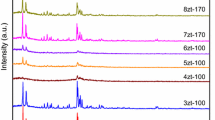
High purity microporous 13X zeolitic materials with octahedral microstructure were successfully synthesized by using Yunnan’s natural halloysite as silica and aluminum sources through hydrothermal method in alkaline media. The effects of various factors such as the molar ratio of SiO2/Al2O3, alkalinity, crystallization time and crystallization temperature were investigated. In addition, the characteristics of its structure and porosity were studied. The optimum synthesis conditions were SiO2/Al2O3 molar ratio of 4.3, NaOH solution concentration of 2 mol/L, aging time at room temperature 12 h and crystallization temperature of 100 °C/8 h. The optimum 13X zeolitic materials were fully characterized by chemical analysis, X-ray diffraction, field emission scanning electron microscopy, thermogravimetry and N2 adsorption–desorption. The as-synthesized 13X zeolitic products exhibited high thermal stability and Langmuir surface area of up to 726 m2/g, value much higher than elsewhere reported.










Similar content being viewed by others
References
D.W. Breck, Zeolite Molecular Sieves: Structure, Chemistry and Uses (Wiley, New York, 1974)
M. Guisnet, F.R. Ribeiro, Zeolitos: um nanomundo ao serviço da catalise, 1a Ed ed. (Fundacao Calouste Gulbenkian, Lisboa, 2004)
D. Novembre, B. Di Sabatino, D. Gimeno, M. Garcia-Valles, S. Martinez-Manent, Micropor. Mesopor. Mater. 75, 1 (2004)
T.S. Jamil, H.S. Ibrahim, I.H. Abd El-Maksoud, S.T. El-Wakeel, Desalination 258, 34 (2010)
R.M. Barrer, Zeolites and Clay Minerals as Sorbents and Molecular Sieves (Academic Press, London, 1978)
C. Haidouti, Sci. Total Environ. 208, 105 (1997)
S.K. Ouki, M. Kavannagh, Waste Manag. Res. 15, 383 (1997)
M.I. Panayotowa, Waste Manag. 23, 135 (2003)
K.D. Mondale, R.M. Carland, F.F. Aplan, Miner. Eng. 8, 535 (1995)
D. Leppert, Eng. Miner. J. 42, 604 (1990)
S. Chandrasekhar, P.N. Pramada, J. Porous Mater. 6, 283 (1999)
R. Terzanoa, M. Spagnuoloa, L. Medicib, F. Tateoc, P. Ruggieroa, Appl. Clay Sci. 29, 99 (2005)
T. Wajima, Y. Ikegami, Ceram. Int. 35, 3983 (2009)
M. Mezni, A. Hamzaoui, N. Hamdi, E. Srasra, Appl. Clay Sci. 52, 209 (2011)
R. Ruiz, C. Banco, C. Pesquera, F. Gonzalez, I. Benito, J.L. Lopez, Appl. Clay Sci. 12, 73 (1997)
S.R. Lee, Y.S. Han, J.H. Choy, Solid State Ionics 151, 343 (2002)
Y.M. Lvov, D.G. Shchukin, H. Mohwald, R.R. Price, ACS Nano 2, 814 (2008)
P. Luo, Y.F. Zhao, B. Zhang, J.D. Liu, Y. Yang, J.F. Liu, Water Res. (2009) doi:10.1016/j.watres.2009.10.042
S.J. Kang, K. Egashira, A. Yoshida, Appl. Clay Sci. 13, 117 (1998)
C.A. Ríos, C.D. Williams, Fuel 87, 2482 (2008)
F.G. Colina, J. Llorens, Microporous Mesoporous Mater. 100, 302 (2007)
A. de Lucas, M.A. Uguina, I. Covian, L. Rodriguez, Ind. Eng. Chem. Res. 32, 1645 (1993)
Z.J. Wei, C.Y. Wang, H. Liu, S.W. Zou, Z. Tong, J. Appl. Polym. Sci. (2012). doi:10.1002/app.36456
Y.S. Liu, H.M. Nan, Q. Cai, H.D. Li, J. Appl. Polym. Sci. (2012). doi:10.1002/app.34125
A. de Lucas, M.A. Uguina, I. Covian, L. Rodriguez, Ind. Eng. Chem. Res. 31, 2134 (1992)
H. Lechert, H. Kacirek, Zeolites 13, 192 (1993)
M.S. Joshi, V.V. Joshi, A.L. Choudhari, M.W. Kasture, Mater. Chem. Phys. 48, 160 (1997)
E.M. Flanigen, in Structural Analysis by Infrared Spectroscopy, ed. by J.A. Rabo (American Chemical Society, Washington, DC, 1976), pp. 80–117
R. Terzano, M. Spagnuolo, L. Medici, F. Tateo, Appl. Geochem. 21, 993 (2006)
T.I. Titova, L.S. Kosheleva, H. Kosslick, J. Mol. Struct. 349, 473 (1995)
J.W. Ward, J. Catal. 10, 34 (1968)
J.E. Sponer, Z. Sobalik, J. Phys. Chem. B105, 8285 (2001)
H. Lechert, H. Kacirek, Zeolites 11, 720 (1991)
Acknowledgments
The financial supports from Chinese land resource ministry project are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, C., Alshameri, A., Yan, C. et al. Characteristics and evaluation of synthetic 13X zeolite from Yunnan’s natural halloysite. J Porous Mater 20, 587–594 (2013). https://doi.org/10.1007/s10934-012-9631-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-012-9631-9