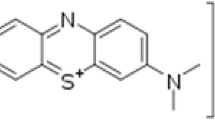
Abstract
Two different mesoporous silicas (MPS) were synthesized by hydrothermal treatment in NaOH solution of two SiO2 sources. These were microporous silica (MicroPS) derived from selectively acid leached metakaolinite and tetraethylorthosilicate (TEOS). The hydrothermal syntheses of the MPSs were performed at a ratio of SiO2/cetyltrimethyl- ammonium bromide (CTABr)/NaOH/H2O = 1/0.1/0.3/150. The specific surface areas (S BET) of the MPSs from MicroPS (MPS(M)) and TEOS (MPS(T)) were 1070 and 1020 m2/g, respectively. Composites of MPS (75 mass%) with TiO2 (25 mass%) were prepared using both SiO2 and two commercial TiO2 powders, P25 and ST-01. The adsorption–desorption behavior of methylene blue (MB) by the four resulting composites and the two MPSs alone was unique in showing partially reversible behavior. The maximum MB adsorption, observed in the composite of ST-01 with MPS(M), designated (S/M), was 0.034 mmol/g. The rates of MB adsorption in the dark and photodecomposition under UV illumination were considerably different for the four composites and two TiO2 powers, and followed the order ST-01 < S/T < P25 < P/T ≈ P/M ≪ S/M. The removal rate of MB by the composite S/M by adsorption and photodecomposition was further enhanced by heating at 700 °C. Direct photodecomposition of MB without adsorption in the dark was greatly enhanced in the composites, especially in that composed of MPS(M) and ST-01.










Similar content being viewed by others
References
T. Yanagisawa, T. Shimizu, K. Kuroda, C. Kato, Bull. Chem. Soc. Japan. 63, 988 (1990)
C.T. Kresge, M.E. Loenowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, Nature 359, 710 (1992)
A. Sayari, Stud. Surf. Sci. Catal. 102, 1 (1996)
A.E.C. Palmqvist, Curr. Opin. Colloids Interface Sci. 8, 145 (2003)
K. Okada, K. J. D. MacKenzie, in Nanomaterials from research to applications, ed. by H. Hosono, Y. Mishima, H. Takezoe, K. J. D. MacKenzie (Elsevier, Amsterdam, 2006), p. 349
K. Okada, K.J.D. MacKenzie, Current Topics Colloid Interf. Sci. 7, 1 (2006)
K. Okada, N. Yamamoto, Y. Kameshima, A. Yasumori, J. Coll. Interf. Sci. 262, 179 (2003)
K. Okada, A. Shimai, T. Takei, S. Hayashi, A. Yasumori, K.J.D. MacKenzie, Micropor. Mesopor. Mater. 21, 289 (1998)
C.D. Madhusoodana, Y. Kameshima, A. Yasumori, K. Okada, Clay Sci. 11, 369 (2001)
C.D. Madhusoodana, Y. Kameshima, A. Nakajima, K. Okada, T. Kogure, K.J.D. MacKenzie, J. Colloid Interf. Sci. 297, 724 (2006)
K. Okada, H. Yoshizaki, Y. Kameshima, A. Nakajima, K.J.D. MacKenzie, J. Porous. Mater. 17, 19 (2010)
T. Linssen, F. Meers, K. Cassiers, P. Cool, E.F. Vansant, J. Phys. Chem. B107, 8599 (2003)
A. Fujishima, X.T. Zhang, D.A. Tryk, Surf. Sci. Reports 63, 515 (2008)
M. Pera-Titus, V. Garcia-Molina, M.A. Banos, J. Gimenez, S. Esplugas, Appl. Catal. B 47, 219 (2004)
N.B. Lihitkar, M.K. Abyamesh, V. Samuel, R. Pasricha, S.W. Gosavi, S.K.K. Kulkarni, J. Coll. Interf. Sci. 314, 310 (2007)
B.J. Aronson, C.F. Blanford, A. Stein, Chem. Mater. 9, 2842 (1997)
K. Inumaru, T. Kasahara, M. Yasui and S. Yamanaka, Chem. Comm. 2131 (2005)
S. Brunauer, P.H. Emmet, E. Teller, J. Am. Chem. Soc. 60, 309 (1938)
E.P. Barrett, L.G. Joyner, P.P. Halenda, J. Am. Chem. Soc. 73, 373 (1951)
S. Wang, H. Li, Micropor. Mesopor. Mater. 97, 21–26 (2006)
Y.S. Ho, G. McKay, J. Environ. Sci. Health A34, 1179 (1999)
E. Tutem, R. Apak, G.F. Unal, Water Res. 32, 2315 (1998)
G. McKay, Chem. Eng. J. 27, 187 (1983)
S.H. Chien, W.R. Clayton, Soil Sci. Soc. Am. J. 44, 265 (1980)
L.C. Juang, C.C. Wang, C.K. Lee, Chemosphere 64, 1920 (2006)
Z. Wu, L. You, H. Ziang, Y. Jiang, J. Colloid Interf. Sci. 303, 346 (2006)
K.F. Lam, K.L. Yeung, G. McKay, Micropor. Mesopor. Mater. 100, 191 (2007)
K. Okada, T. Yanagisawa, Y. Kameshima, A. Nakajima, Mater. Res. Bull. 42, 1921 (2007)
K. Okada, N. Yamamoto, Y. Kameshima, A. Yasumori, K.J.D. MacKenzie, J. Am. Ceram. Soc. 84, 1591 (2001)
K. J. D. MacKenzie, M. E. Smith, Multinuclear solid-state NMR of inorganic materials (Pergamon Materials Series, vol. 6, Pergamon, Oxford, 2002)
Acknowledgments
TEM observation was performed at the Center for Advanced Materials Analysis of Tokyo Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okada, K., Yoshizawa, A., Kameshima, Y. et al. Adsorption and photocatalytic properties of TiO2/mesoporous silica composites from two silica sources (acid-leached kaolinite and Si-alkoxide). J Porous Mater 18, 345–354 (2011). https://doi.org/10.1007/s10934-010-9384-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-010-9384-2